A review of hydrophobic modification of chitosan for enhanced transdermal drug delivery and skin repair
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Chitosan (CS), a linear aminopolysaccharide derived from chitin, exhibits poor solubility in neutral, alkaline, and organic media. This review systematically summarizes recent advances in hydrophobic modification strategies (aldehyde grafting, acylation, and etherification) for CS and their applications in transdermal drug delivery and skin repair. Aldehyde grafting and acylation reactions selectively modified the -NH2 groups of CS under mild conditions. In contrast, etherification was non selective, yielding higher degrees of substitution up to 1.91. Hydrophobically modified chitosan (HM-CS) exhibited enhanced antioxidant properties, antibacterial and antifungal activities, transdermal permeability, and greater emulsion stability compared with unmodified CS. HM-CS self-aggregated into particles with diameters ranging from 80 to 360 nm, which effectively encapsulated and delivered oil-soluble bioactive compounds. Notably, HM-CS-stabilized microemulsions achieved curcumin loading of 3.41 ± 0.13 mg/mL, which was ≈30,000 times its water solubility. Additionally, these microemulsions achieved a cumulative transdermal delivery of 297.30 ± 18.30 μg/cm2 within 24 h in the studied models. HM-CS-stabilized hydrogels accelerated wound re-epithelialization within 5 days, and achieved complete (100 %) wound contraction by day 10, driven by enhanced angiogenesis and collagen deposition. The modification parameters were correlated with self-aggregation behavior and biological efficacy. These findings provide a framework for optimizing CS-based carriers in biomedical applications. This review highlights the potential of HM-CS in transdermal drug delivery and identifies key challenges, including scalable synthesis and clinical validation, to guide future research.
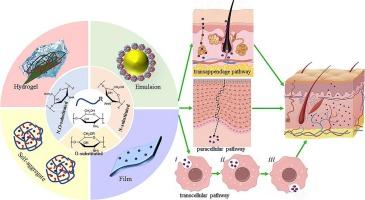
壳聚糖疏水改性增强经皮给药和皮肤修复的研究进展
壳聚糖(CS)是一种从几丁质中提取的线性氨基多糖,在中性、碱性和有机介质中具有较差的溶解性。本文系统综述了近年来CS疏水修饰策略(醛接枝、酰化和醚化)及其在经皮给药和皮肤修复中的应用。醛接枝和酰化反应在温和条件下选择性修饰了CS的-NH2基团。相比之下,醚化是非选择性的,产生更高的取代度,最高可达1.91。疏水改性壳聚糖(HM-CS)与未改性壳聚糖相比,具有更强的抗氧化性能、抗菌和抗真菌活性、透皮渗透性和乳液稳定性。HM-CS自聚集成直径为80 ~ 360 nm的颗粒,可有效包裹和递送油溶性生物活性化合物。值得注意的是,hm - cs稳定微乳的姜黄素负载为3.41±0.13 mg/mL,是其水溶性的约3万倍。此外,这些微乳在24小时内达到297.30±18.30 μg/cm2的累积透皮递送。hm - cs稳定的水凝胶在5天内加速了伤口的再上皮化,并在血管生成和胶原沉积增强的推动下,在第10天实现了伤口完全(100%)收缩。修饰参数与自聚集行为和生物功效相关。这些发现为优化生物医学应用中基于cs的载体提供了一个框架。这篇综述强调了HM-CS在经皮给药方面的潜力,并确定了关键挑战,包括可扩展的合成和临床验证,以指导未来的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


