Continuous cultivation of defined mixed bacterial cultures for upcycling polyurethane and polyethylene terephthalate monomers to polyhydroxyalkanoates
IF 7.4
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Prostate cancer is an androgen-dependent tumor, and androgen deprivation therapy (ADT) is an important treatment modality. Relugolix is the first oral GnRH receptor antagonist, which has been approved for clinical use. However, its oral formulation requires daily administration, leading to poor patient adherence and suboptimal treatment outcomes for long-term users. To address these issues, we developed a long-acting micronized formulation of relugolix long-acting microcrystalline formulation that is administered once every 28 days, and systematically evaluated its pharmacokinetics (PK), pharmacodynamics (PD), and safety profile in male Beagle dogs. In this study, we established a high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method for blood concentration detection, used a highly specific and stable ELISA method to measure serum testosterone levels, and assessed the safety of the drug in male Beagle dogs. The HPLC-MS/MS method showed good linearity (R2=0.9991). The intra-day precision varied between 2.8% and 10.9%, while the inter-day precision ranged from 1.6% to 5.4%. In terms of accuracy, the intra-day values ranged from -7.3% to -3.2%, and the inter-day accuracy ranged from -10.2% to 0.8%. Furthermore, key parameters such as stability, extraction recovery, and matrix effects met the FDA bioanalytical method validation guidelines. Pharmacokinetic studies showed that the AUC and Tmax exhibited significant sustained-release characteristics, and the half-life was notably extended compared to Orgovyx. Pharmacodynamic analysis showed a significant negative correlation between blood drug concentration and testosterone suppression (testosterone levels began to decline after administration, and castration levels were maintained between 504-1008 hours). Safety assessments revealed that although no treatment-related deaths or significant weight changes were observed, some hematological parameters (RBC, HGB increases), liver function indicators (ALT, AST increases), and triglyceride (TG) levels showed statistically significant changes (p<0.05). This suggests potential concerns regarding hemodynamic effects, hepatotoxicity, and lipid metabolism
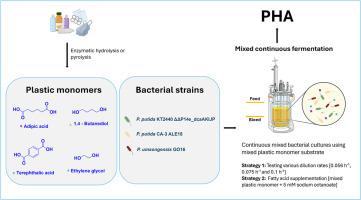
连续培养的定义的混合细菌培养升级回收聚氨酯和聚对苯二甲酸酯单体到聚羟基烷酸酯
前列腺癌是一种雄激素依赖性肿瘤,雄激素剥夺治疗(ADT)是一种重要的治疗方式。Relugolix是第一个口服GnRH受体拮抗剂,已被批准用于临床。然而,其口服制剂需要每天给药,导致患者依从性差,长期使用者的治疗结果不理想。为了解决这些问题,我们开发了一种长效微晶relugolix长效微晶制剂,每28天给药一次,并系统评估了其在雄性Beagle犬中的药代动力学(PK)、药效学(PD)和安全性。本研究建立了高效液相色谱-串联质谱(HPLC-MS/MS)血药浓度检测方法,采用特异性高、稳定性好的ELISA法测定血清睾酮水平,并对该药物在雄性Beagle犬体内的安全性进行了评估。HPLC-MS/MS方法线性良好(R2=0.9991)。日内精度在2.8% ~ 10.9%之间,日内精度在1.6% ~ 5.4%之间。准确度方面,日内值范围为-7.3% ~ -3.2%,日内值范围为-10.2% ~ 0.8%。此外,稳定性、萃取回收率和基质效应等关键参数符合FDA生物分析方法验证指南。药动学研究表明,AUC和Tmax具有显著的缓释特性,半衰期较Orgovyx明显延长。药效学分析显示血药浓度与睾酮抑制呈显著负相关(给药后睾酮水平开始下降,去势水平维持在504-1008小时)。安全性评估显示,虽然没有观察到与治疗相关的死亡或明显的体重变化,但一些血液学参数(RBC、HGB升高)、肝功能指标(ALT、AST升高)和甘油三酯(TG)水平显示有统计学意义的变化(p<0.05)。这提示潜在的血流动力学影响、肝毒性和脂质代谢问题
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Degradation and Stability
化学-高分子科学
CiteScore
10.10
自引率
10.20%
发文量
325
审稿时长
23 days
期刊介绍:
Polymer Degradation and Stability deals with the degradation reactions and their control which are a major preoccupation of practitioners of the many and diverse aspects of modern polymer technology.
Deteriorative reactions occur during processing, when polymers are subjected to heat, oxygen and mechanical stress, and during the useful life of the materials when oxygen and sunlight are the most important degradative agencies. In more specialised applications, degradation may be induced by high energy radiation, ozone, atmospheric pollutants, mechanical stress, biological action, hydrolysis and many other influences. The mechanisms of these reactions and stabilisation processes must be understood if the technology and application of polymers are to continue to advance. The reporting of investigations of this kind is therefore a major function of this journal.
However there are also new developments in polymer technology in which degradation processes find positive applications. For example, photodegradable plastics are now available, the recycling of polymeric products will become increasingly important, degradation and combustion studies are involved in the definition of the fire hazards which are associated with polymeric materials and the microelectronics industry is vitally dependent upon polymer degradation in the manufacture of its circuitry. Polymer properties may also be improved by processes like curing and grafting, the chemistry of which can be closely related to that which causes physical deterioration in other circumstances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


