Levofloxacin-induced seizure susceptibility involves both enhanced glutamatergic and impaired GABAergic synaptic function
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Levofloxacin (LVFX)-associated seizures are thought to arise from disrupted excitatory-inhibitory balance, but the underlying synaptic mechanisms remain unclear. This study investigated how LVFX alters both glutamatergic and GABAergic transmission to promote neuronal hyperexcitability. We combined in vitro and in vivo approaches using primary cortical neurons treated with LVFX and adult rats administered LVFX. Electrophysiological recordings assessed AMPA receptor (AMPAR)-mediated miniature excitatory postsynaptic currents (mEPSCs) and GABAergic miniature inhibitory postsynaptic currents (mIPSCs). Molecular analyses examined vesicular glutamate transporter 1 (VGluT1) and vesicular GABA transporter (VGAT) expression, along with AMPAR subunit GluA1 trafficking dynamics. Seizure susceptibility was evaluated using magnesium-free conditions in vitro and pentylenetetrazol challenge in vivo. LVFX treatment significantly increased mEPSC frequency and amplitude while decreasing mIPSC frequency, indicating enhanced excitatory and suppressed inhibitory synaptic transmission. These changes were accompanied by upregulated VGluT1 and downregulated VGAT protein expression. The drug specifically altered GluA1 trafficking by decreasing internalization and promoting recycling to the plasma membrane. These synaptic modifications resulted in heightened seizure susceptibility, with LVFX-treated neurons showing earlier epileptiform discharges and pretreated animals exhibiting significantly reduced seizure latency. LVFX lowers seizure threshold by simultaneously enhancing glutamatergic transmission and suppressing GABAergic inhibition, providing a mechanistic basis for its pro-convulsant effects.
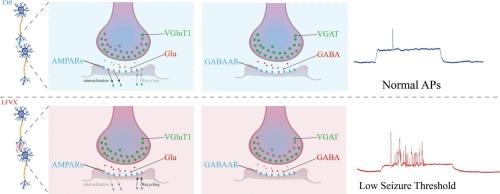
左氧氟沙星诱导的癫痫易感性涉及谷氨酸能增强和gaba能突触功能受损
左氧氟沙星(LVFX)相关的癫痫发作被认为是由兴奋-抑制平衡被破坏引起的,但潜在的突触机制尚不清楚。本研究探讨了LVFX如何改变谷氨酸能和gaba能的传递,从而促进神经元的高兴奋性。我们采用体外和体内相结合的方法,用LVFX治疗初级皮质神经元,并用LVFX治疗成年大鼠。电生理记录评估了AMPA受体(AMPAR)介导的微型兴奋性突触后电流(mEPSCs)和gaba能微型抑制性突触后电流(mIPSCs)。分子分析检测了泡状谷氨酸转运蛋白1 (VGluT1)和泡状GABA转运蛋白(VGAT)的表达,以及AMPAR亚基GluA1的转运动态。在体外无镁条件下和体内戊四唑激发下评估癫痫易感性。LVFX治疗显著增加了mEPSC频率和幅度,降低了mIPSC频率,表明兴奋性突触传递增强,抑制性突触传递受到抑制。这些变化伴随着VGluT1的上调和VGAT蛋白表达的下调。该药物通过减少内化和促进质膜的再循环,特异性地改变了GluA1的贩运。这些突触修饰导致癫痫易感性增加,lvfx处理的神经元表现出更早的癫痫样放电,而预处理的动物表现出明显减少的癫痫潜伏期。LVFX通过同时增强谷氨酸能传递和抑制gaba能抑制来降低癫痫发作阈值,为其促惊厥作用提供了机制基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


