Optimal stimulation site and fiber tracts in subthalamic deep brain stimulation for Meige syndrome
IF 8.4
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
Abstract
Background
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) has emerged as an effective therapy for Meige syndrome (MS). However, the optimal stimulation site within STN and the most effective stimulation fiber tracts have not been investigated.
Methods
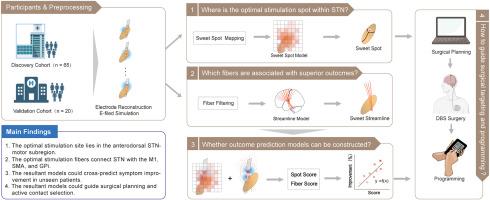
Based on the discovery cohort (n = 65), we first identified the optimal stimulation site within the STN using the sweet spot mapping method. Second, we screened for the fiber tracts accounting for optimal clinical outcomes by the fiber filtering approach. Third, based on the above findings, we constructed outcome prediction models and estimated their predictive performance in the discovery cohort and an independent validation cohort (n = 20). Finally, we introduced two prospective cases to illustrate if and how the optimal stimulation site and fiber tracts could facilitate precise electrode targeting and postoperative programming.
Results
The optimal stimulation site was mapped to the anterodorsal portion of the STN-motor subregion. Superior STN-DBS outcomes were positively correlated with stimulation of the fibers projecting to the primary motor cortices, the supplementary motor areas, and the globus pallidus internus. Notably, spatial overlap between individual stimulation volumes and the resultant sweet spot or fiber filtering models could cross-predict symptom improvement in out-of-model patients. Moreover, the models could guide electrode implantation and active contact selection in prospective cases.
Conclusion
Our study underscores the potential of optimizing stimulation sites and fibers to predict clinical improvement, and provides new insights into the ongoing efforts of precise surgical targeting and computer-assisted DBS programming.
Meige综合征丘脑下深部脑刺激的最佳刺激部位和纤维束
颅底核深部脑刺激(DBS)已成为治疗Meige综合征(MS)的有效方法。然而,STN内的最佳刺激部位和最有效的刺激纤维束尚未得到研究。方法基于发现队列(n = 65),我们首先使用甜点映射方法确定了STN内的最佳增产部位。其次,我们通过纤维过滤方法筛选具有最佳临床结果的纤维束。第三,基于上述发现,我们构建了结局预测模型,并对其在发现队列和独立验证队列(n = 20)中的预测性能进行了估计。最后,我们介绍了两个前瞻性病例来说明最佳刺激部位和纤维束是否以及如何促进精确的电极靶向和术后规划。结果最佳刺激部位定位于stn -运动亚区的前嗅部。良好的STN-DBS结果与投射到初级运动皮质、辅助运动区和内苍白球的纤维的刺激呈正相关。值得注意的是,个体刺激量与产生的最佳点或纤维过滤模型之间的空间重叠可以交叉预测模型外患者的症状改善。此外,该模型还可以指导前瞻性病例的电极植入和主动接触选择。结论我们的研究强调了优化刺激部位和纤维预测临床改善的潜力,并为正在进行的精确手术靶向和计算机辅助DBS编程提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Brain Stimulation
医学-临床神经学
CiteScore
13.10
自引率
9.10%
发文量
256
审稿时长
72 days
期刊介绍:
Brain Stimulation publishes on the entire field of brain stimulation, including noninvasive and invasive techniques and technologies that alter brain function through the use of electrical, magnetic, radiowave, or focally targeted pharmacologic stimulation.
Brain Stimulation aims to be the premier journal for publication of original research in the field of neuromodulation. The journal includes: a) Original articles; b) Short Communications; c) Invited and original reviews; d) Technology and methodological perspectives (reviews of new devices, description of new methods, etc.); and e) Letters to the Editor. Special issues of the journal will be considered based on scientific merit.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


