Hydrogenation of CO2 to CH3OH on the Cu-ZnO-BaTiO3 catalysts: The electronic metal-support interaction (EMSI) induces the upshift of the d-band center of Cu atoms in Cu-based catalysts
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
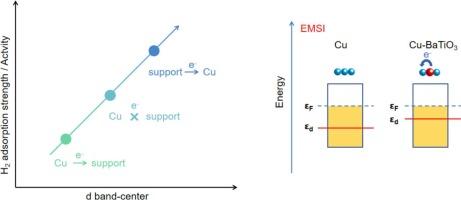
The study of catalysts for the efficient hydrogenation of CO2 to methanol is imperative for the reduction of atmospheric CO2 content. In this paper, Cu-based catalysts with different supports loadings were prepared by deposition precipitation method. The purpose of this study was to investigate the reasons for the shift of the d-band center of Cu atoms in Cu-based catalysts and its effect on the catalytic activity of CO2 hydrogenation to methanol. In situ XPS and CO-DRIFTS characterization demonstrated that the content of coordinatively unsaturated Cu0 step-edge sites is identified as a structural descriptor for the catalyst d-band center.
Electrons transfer from supports to Cu (EMSI) favored the formation of coordinatively unsaturated Cu0 step-edge sites, which in turn resulted in the upshift of the catalyst d-band center. The direction and degree of electrons transfer between the supports and Cu were determined by the supports’ energy-band structure. Experiments involving H2-TPD and catalytic activity demonstrated that the upshift of the d-band center enhanced the adsorption strength of the catalyst for H2 and improved the catalytic activity.
Cu- zno - batio3催化剂上CO2加氢生成CH3OH:电子金属-载体相互作用(EMSI)诱导Cu基催化剂中Cu原子d波段中心的上移
研究二氧化碳高效加氢制甲醇催化剂对降低大气中二氧化碳含量具有重要意义。采用沉积沉淀法制备了不同载体负载的铜基催化剂。本研究的目的是探讨Cu基催化剂中Cu原子d带中心移位的原因及其对CO2加氢制甲醇催化活性的影响。原位XPS和CO-DRIFTS表征表明,配位不饱和Cu0阶边位点的含量被确定为催化剂d带中心的结构描述符。电子从载体转移到Cu (EMSI)有利于形成配位不饱和Cu0阶边位,从而导致催化剂d带中心的上移。支撑物的能带结构决定了支撑物与Cu之间电子转移的方向和程度。H2- tpd和催化活性实验表明,d波段中心的上移增强了催化剂对H2的吸附强度,提高了催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


