Ultra trace mercury detection using a DNA aptamer sensor based on the electrochemical transformation of metal-organic frameworks
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Mercury pollution has always been a concern due to its high toxicity and persistence. In this study, we designed an aptamer-based sensor relying on lanthanide metal-organic framework and in-situ generation of active substances through electrochemical conversion. Firstly, a lanthanide metal-organic framework with rod-shaped structure was successfully synthesized, which has higher conductivity and stability compared to traditional MOFs. By possessing the substantial specific surface area and profusion of active sites offered by rod-shaped Gd4-MOF, gold nanoparticles (AuNPs) were deposited for the connection of tetrahedron DNA nanostructure (TDN). The active substances generated by electrochemical conversion has a more stringent arrangement and better response current compared to ordinary active substances, which can be used for ultra trace detection of mercury with higher sensitivity. After mercury added, DNA and mercury specifically bind to form thymine–Hg–thymine (T-Hg-T) structures, which cause changes in response current through conformational changes. The results demonstrated that a pronounced linear correlation between the DPV response and mercury concentration was observable, with a linear range of 1 fM to 200 fM and a detection limit of 0.66 fM. This sensor is successfully applied to detect the mercury content in marine products, so it has great application prospects for preventing and controlling mercury pollution.
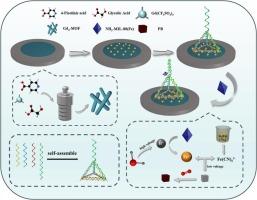
基于金属-有机框架电化学转化的DNA适体传感器检测超痕量汞
汞的高毒性和持久性一直是人们关注的问题。在这项研究中,我们设计了一个基于适配体的传感器,依靠镧系金属有机框架和通过电化学转化原位生成活性物质。首先,成功合成了一种具有棒状结构的镧系金属有机骨架,与传统mof相比,该骨架具有更高的导电性和稳定性。利用棒状Gd4-MOF提供的大量比表面积和丰富的活性位点,沉积金纳米颗粒(AuNPs)用于连接四面体DNA纳米结构(TDN)。与普通活性物质相比,电化学转化产生的活性物质排列更严格,响应电流更好,可用于汞的超痕量检测,灵敏度更高。加入汞后,DNA与汞特异性结合形成胸腺嘧啶-氢-胸腺嘧啶(T-Hg-T)结构,通过构象变化引起响应电流的变化。结果表明,DPV响应与汞浓度之间存在显著的线性关系,线性范围为1 ~ 200 fM,检出限为0.66 fM。该传感器已成功应用于海洋产品中汞含量的检测,在汞污染防治方面具有很大的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


