Mitigating SARS-CoV-2–exacerbated neuroinflammation and Alzheimer's pathology: Synthesis of quinoline-based dual inhibitors targeting cysteine and aspartyl proteases
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Viral and neurodegenerative proteases, such as the cysteine protease and aspartyl protease, offer strategic targets in a multitarget therapeutic approach for Alzheimer's disease, especially when viral infection may exacerbate neurological degeneration. To establish a multitarget therapeutic for treating Alzheimer's disease, we chose β-secretase (BACE-1), an aspartyl protease, and the SARS-CoV-2 main protease (Mpro), a cysteine protease, as dual targets. In search of BACE-1 and Mpro inhibitors, a set of novel quinoline-4-carboxamide derivatives (2a-k, 3a-c, 4a-d, 5a-c, 6a-c) was synthesized using the Suzuki coupling reaction in good to excellent yields. All the synthesized compounds were screened in vitro for their potential inhibitory activities against two proteases. Compounds 2e, 6b, and 6c emerged as dual inhibitors of both proteases. Furthermore, a kinetic study revealed the mechanism of BACE-1 inhibition by compound 6c. Safety profiling of the most potent dual inhibitor 6c was performed via an in vivo acute cytotoxicity assay on Swiss albino mice. Molecular docking studies were conducted against aspartyl protease (2HM1) and cysteine protease (6XHM) to elucidate the binding interaction of synthesized quinoline derivatives. The compound 6c exhibited strong binding affinities through multiple hydrogen bonds, π-Sulfur and π-π stacking interactions, within key subpockets of both targets, supporting inhibitory potential. These studies revealed that the lead compound 6c could be a good drug candidate with further structural modifications.
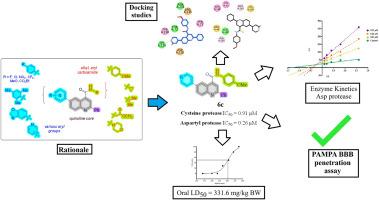
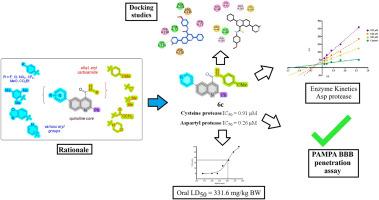
缓解sars - cov -2加重的神经炎症和阿尔茨海默病病理:合成以喹啉为基础的半胱氨酸和天冬氨酸蛋白酶双抑制剂
病毒和神经退行性蛋白酶,如半胱氨酸蛋白酶和天冬氨酸蛋白酶,为阿尔茨海默病的多靶点治疗方法提供了战略靶点,特别是当病毒感染可能加剧神经退行性变时。为了建立治疗阿尔茨海默病的多靶点疗法,我们选择了天冬氨酸蛋白酶β-分泌酶(BACE-1)和半胱氨酸蛋白酶SARS-CoV-2主蛋白酶(Mpro)作为双靶点。为了寻找BACE-1和Mpro抑制剂,采用Suzuki偶联反应合成了一组新的喹啉-4-羧胺衍生物(2a-k, 3a-c, 4a-d, 5a-c, 6a-c),收率较高。所有合成的化合物对两种蛋白酶的潜在抑制活性进行体外筛选。化合物2e、6b和6c是两种蛋白酶的双重抑制剂。此外,动力学研究揭示了化合物6c抑制BACE-1的机制。通过瑞士白化病小鼠体内急性细胞毒性试验,对最有效的双抑制剂6c进行了安全性分析。通过与天冬氨酸蛋白酶(2HM1)和半胱氨酸蛋白酶(6XHM)的分子对接研究,阐明合成的喹啉衍生物的结合相互作用。化合物6c在两个靶标的关键子口袋内通过多个氢键、π-硫和π-π堆叠相互作用表现出很强的结合亲和力,支持抑制潜力。这些研究表明,通过进一步的结构修饰,先导化合物6c可能是一个很好的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


