Aurora A degradation by HSP90 interactome-mediating PROTACs in A549 and paclitaxel-resistant A549 cells
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Lung cancer has one of the higher incidence and mortality rates worldwide. The development of drug resistance poses a significant challenge to lung cancer treatment. Aurora A kinase, a member of the Aurora family of proteins, has been identified as a key regulator of the cell cycle and mitotic spindle assembly, and overexpression is frequently observed in tumors. Kinase-independent oncogenic functions may be responsible for low clinical response rates, which are difficult to target the conventional small molecules. Targeting both the catalytic and non-catalytic functions of Aurora A may be a viable approach. In this study, we have designed and synthesized a series of novel Aurora A protein degradation-targeted chimeras (Aurora A-PROTACs) based on the HSP90 interactome. Unlike existing Aurora A PROTACs, the new AurAPs series utilizes HSP90, which is highly expressed in tumor cells, as the ligand to recruit the HSP90/E3 ubiquitin ligase complex. AurAPs induced the degradation of the target protein Aurora A by “hijacking” the HSP90/E3 complexes, effectively increasing the targeting of tumors. In vitro biochemical and cellular assays showed that AurAP14 effectively degraded Aurora A kinase, inhibited the proliferation of most human tumor cells and effectively attenuated the development of paclitaxel-resistant lung cancer cells. In addition, AurAP14 significantly inhibited the tumor growth of NSCLC and drug-resistant NSCLC xenograft tumor mice. The results from this study indicate that AurAP14 represents a promising delivery strategy for the sequential elimination of multiple functions of oncogenic proteins and the attenuation of chemotherapy-induced drug resistance.
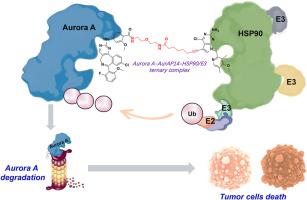
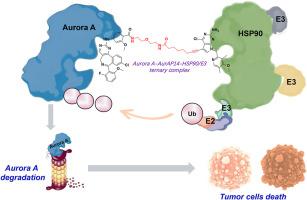
HSP90相互作用体介导的PROTACs在A549和紫杉醇抗性A549细胞中降解Aurora A
肺癌是世界上发病率和死亡率较高的疾病之一。耐药的发展对肺癌的治疗提出了重大挑战。Aurora A激酶是Aurora蛋白家族的一员,已被确定为细胞周期和有丝分裂纺锤体组装的关键调节因子,在肿瘤中经常观察到过表达。不依赖激酶的致癌功能可能导致临床反应率低,这是难以针对传统的小分子。同时针对Aurora A的催化和非催化功能可能是一种可行的方法。在本研究中,我们设计并合成了一系列基于HSP90相互作用组的新型Aurora a蛋白降解靶向嵌合体(Aurora a - protacs)。与现有的Aurora A PROTACs不同,新的AurAPs系列利用在肿瘤细胞中高表达的HSP90作为配体来招募HSP90/E3泛素连接酶复合物。AurAPs通过“劫持”HSP90/E3复合物诱导靶蛋白Aurora A的降解,有效增加肿瘤的靶向性。体外生化和细胞实验表明,AurAP14能有效降解Aurora A激酶,抑制大多数人肿瘤细胞的增殖,并能有效减弱紫杉醇耐药肺癌细胞的发展。此外,AurAP14显著抑制非小细胞肺癌和耐药非小细胞肺癌异种移植瘤小鼠的肿瘤生长。本研究的结果表明,AurAP14是一种有前景的递送策略,可以连续消除致癌蛋白的多种功能,并减弱化疗诱导的耐药。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


