4-substituted (1,2,5-oxadiazol-3-yl)benzamides and -benzene sulfonamides as antiplasmodial agents
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
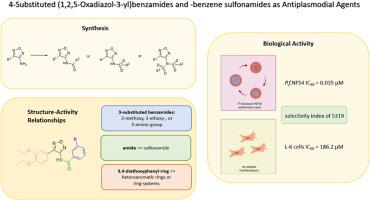
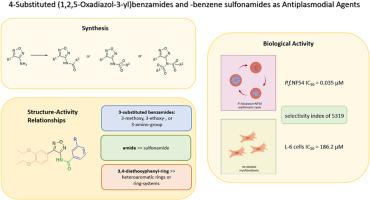
The 1,2,5-oxadiazol MMV665805 from Medicines for Malaria Venture's Malaria Box Project is the lead compound of herein presented study. It exhibits very promising activity against the blood stages of the strain NF54 of Plasmodium falciparum. Twenty-five new derivates were prepared by replacing the initial left-hand side 3,4-diethoxyphenyl ring as well as introducing various benzamido and benzenesulfonamido substituents in position 3 of the furazan. Thereby, an insightful series of valuable structure-activity-relationships was elaborated. Compounds were furthermore analyzed for their compliance with Lipinski's rules for drug-likeness. Additionally, passive permeability was determined experimentally. A 3-amino-N-[4-(3,4-diethoxyphenyl)-1,2,5-oxadiazol-3-yl]benzamide not only complies perfectly with Lipinski's rules, most notably, it possessed excellent antiplasmodial activity against the chloroquine-sensitive strain P. falciparum NF 54 (IC50 = 0.035 μM). Furthermore, it is nearly nontoxic for rat L-6 cells (IC50 = 186.2 μM) resulting in a marvelous selectivity index of 5319.
4-取代(1,2,5-恶二唑-3-基)苯酰胺和-苯磺酰胺作为抗疟原虫药物
1,2,5-恶二唑MMV665805来自Medicines for Malaria Venture’s Malaria Box Project,是本研究的先导化合物。它对恶性疟原虫NF54株血液阶段表现出非常有希望的活性。通过在呋喃嘧啶的3位上引入不同的苯胺和苯磺酸胺取代基,取代了最初的左手边3,4-二氧基苯基环,制备了25个新的衍生物。由此,作者阐述了一系列有价值的结构-活动-关系。进一步分析化合物是否符合Lipinski的药物相似性规则。此外,实验测定了被动渗透率。3-氨基- n -[4-(3,4-二氧基苯基)-1,2,5-恶二唑-3-基]苯甲酰胺不仅完全符合Lipinski规则,而且对氯喹敏感的恶性疟原虫NF - 54具有良好的抗疟原虫活性(IC50 = 0.035 μM)。此外,对大鼠L-6细胞(IC50 = 186.2 μM)几乎无毒,选择性指数为5319。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


