Targeting galectin-4 with glycoconjugates of varying architectures: a multivalency étude with accent on anti-tumor effect and protection against apoptosis
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Galectin-4 (Gal-4) is a protumorigenic protein that strongly participates especially in gastrointestinal cancer and is increasingly recognized as a therapeutic target in gastrointestinal malignancies, where its dysregulated expression contributes to tumor progression and immune modulation. Its abundance and the extent of its involvement in these pathologies are comparable with its much better-studied counterparts, galectin-1 and -3. However, to date, no systematic effort has been made to design efficient defined Gal-4 inhibitors with a potential therapeutic effect. In this work, we present a library of biocompatible multivalent Gal-4 inhibitors with diverse architectures to investigate how different modes of multivalent presentation of a common ligand, lactose, influence the binding affinity to Gal-4. The most efficient scaffold was then loaded with a high-affinity tetrasaccharide ligand of Gal-4, derived from lacto-N-tetraose, to further enhance Gal-4 binding. The resulting glycopolymer exhibited outstanding performance in inhibiting Gal-4-induced apoptosis of T lymphocytes, and, in addition, it efficiently scavenged Gal-4 from the surface of cancer cells. Both of these processes are relevant for tumor immune evasion. Given the high biocompatibility, and low toxicity of the POx carrier, this glycopolymer has a strong potential as a candidate for further development of glycotherapeutics against Gal-4-associated diseases such as gastrointestinal cancer.

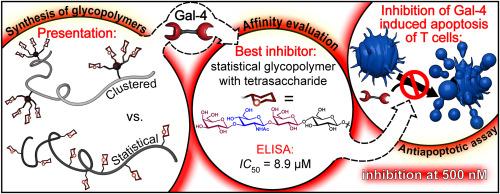
用不同结构的糖缀合物靶向半乳糖凝集素-4:一种以抗肿瘤作用和保护细胞凋亡为重点的多价调制体
半乳糖凝集素-4 (Galectin-4, Gal-4)是一种致瘤蛋白,尤其在胃肠道肿瘤中发挥着重要作用,并日益被认为是胃肠道恶性肿瘤的治疗靶点,其表达失调有助于肿瘤进展和免疫调节。它在这些病理中的丰度和参与程度与研究得更好的同类半乳糖凝集素-1和-3相当。然而,到目前为止,还没有系统的努力来设计具有潜在治疗效果的有效的Gal-4抑制剂。在这项工作中,我们提出了一个具有不同结构的生物相容性多价Gal-4抑制剂库,以研究共同配体乳糖的不同多价呈现模式如何影响与Gal-4的结合亲和力。然后将最有效的支架加载高亲和力的Gal-4四糖配体,该配体来源于乳酸- n -四糖,以进一步增强Gal-4的结合。所得到的糖共聚物在抑制Gal-4诱导的T淋巴细胞凋亡方面表现出优异的性能,此外,它还能有效地清除癌细胞表面的Gal-4。这两个过程都与肿瘤免疫逃避有关。鉴于天花载体的高生物相容性和低毒性,这种糖共聚物具有很强的潜力,可以作为进一步开发针对gal -4相关疾病(如胃肠道癌症)的糖疗法的候选物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


