Improvement of dentin bonding via adhesive monomers with multiple hydrogen bonding moieties
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Despite advancements in bonding techniques, the resin-dentin interface remains the weakest point in dental restorations, susceptible to collagen degradation and methacrylate hydrolysis. One strategy to enhance the resin-dentin interface is to incorporate hydrogen-bonding-rich functional groups into dental adhesive resins, such as 2-ureido-4[1H]-pyrimidinone (UPy). These hydrogen bonds may bridge the adhesive resin and dentin substrate, which contains collagen and hydroxyapatite, as well as form non-covalent crosslinks within the resin. Here, we utilize UPy-functionalized methacrylamides modified with glycol spacers to ensure compatibility with other monomers commonly used in adhesive resins, as well as to promote hydrogen bonding at the resin-dentin interface and within the bulk resin. Three UPy-based methacrylamides: UPy-OPG400-MMA, UPy-OPG230-MMA and UPy-OEG148-MMA were synthesized and incorporated into model methacrylate-based adhesive formulations. Results show that the UPy-methacrylamides enhance polymerization kinetics, biocompatibility, and mechanical performance. However, these improvements and the efficacy of hydrogen-bond formation depend on the flexibility of the glycol spacers. Specifically, resins containing UPy-OPG230-MMA have the most robust hydrogen bonding in aqueous conditions, making them the optimal choice in this study. This selection is further confirmed by micro-tensile bonding strength (TBS) analysis and interfacial characterizations, which shows a significant enhancement in bonding performance when 50 wt% of 2-hydroxyethyl methacrylate (HEMA) is replaced with UPy-OPG230-MMA in a model self-etch adhesive. Overall, this work presents a strategy to enhance dental adhesive performance by incorporating hydrogen-bonding motifs that reinforce both the polymer network and the resin-dentin interface, offering improved durability under clinically relevant conditions.
Statement of Significance:
This study shows that hydrogen bonding interactions improve the overall performance of dental adhesives. Adhesive monomers with the 2-ureido-4[1H]-pyrmidinone (UPy) group facilitate significant hydrogen bonding interactions both within the adhesive as well as between the adhesive and an external substrate (here, dentin). This results in improved mechanical integrity of the adhesive (e.g. strength and integrity of the bonding) and impacts critical biomaterial properties such as biocompatibility. This work is significant as prior demonstrations utilizing UPy functionalities for enhanced adhesion require organic solvents (e.g. DMSO) that are incompatible with in situ, dental materials applications. Here we synthesize UPy-functionalized monomers that are miscible in aqueous solvents (water, ethanol) and compatible with comonomers used in dental materials applications.
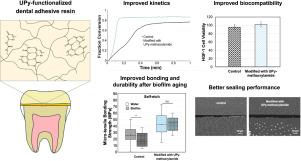
用多氢键基团的粘结单体改善牙本质的粘结。
尽管粘接技术取得了进步,但树脂-牙本质界面仍然是牙齿修复中最薄弱的环节,容易受到胶原蛋白降解和甲基丙烯酸酯水解的影响。一种增强树脂-牙本质界面的策略是将富含氢键的官能团加入到牙科粘合剂树脂中,如2-脲基-4[1H]-嘧啶酮(UPy)。这些氢键可以桥接粘合剂树脂和含有胶原蛋白和羟基磷灰石的牙本质基质,也可以在树脂内部形成非共价交联。在这里,我们利用乙二醇间隔剂修饰的upy功能化甲基丙烯酰胺来确保与粘合树脂中常用的其他单体的相容性,并促进树脂-牙本质界面和大块树脂内部的氢键。合成了三种upy基甲基丙烯酰胺:UPy-OPG400-MMA、UPy-OPG230-MMA和UPy-OEG148-MMA,并将其掺入模型甲基丙烯酸酯基粘合剂配方中。结果表明,upy -甲基丙烯酰胺提高了聚合动力学、生物相容性和力学性能。然而,这些改进和氢键形成的有效性取决于乙二醇间隔剂的柔韧性。具体来说,含有UPy-OPG230-MMA的树脂在水溶液条件下具有最强大的氢键,使其成为本研究的最佳选择。微拉伸结合强度(μTBS)分析和界面表征进一步证实了这一选择,表明当UPy-OPG230-MMA取代50 wt%的2-羟乙基甲基丙烯酸酯(HEMA)时,模型自蚀刻胶的结合性能显著增强。总的来说,这项工作提出了一种策略,通过结合氢键基元来增强聚合物网络和树脂-牙本质界面,从而提高临床相关条件下的耐久性。意义声明:本研究显示了氢键相互作用如何改善牙科胶粘剂的整体性能。具有2-脲基-4[1H]-嘧啶酮(UPy)基团的粘合剂单体促进了粘合剂内部以及粘合剂与外部底物(此处为牙本质)之间的显著氢键相互作用。这提高了粘合剂的机械完整性(例如,粘合的强度和完整性),并影响了关键的生物材料特性,如生物相容性。这项工作是重要的,因为先前的演示利用UPy功能来增强附着力需要有机溶剂(例如DMSO),而有机溶剂与原位牙科材料应用不相容。在这里,我们合成了upy功能化的单体,这些单体可以在水溶剂(水,乙醇)中混溶,并与牙科材料中使用的共聚物相容。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


