A pH-responsive polycarbonate nanoplatform enables sequential drug release for enhanced apoptotic cascade synergy in non-small cell lung cancer therapy
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
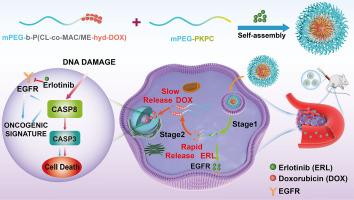
Tumor heterogeneity poses formidable challenges to effective cancer therapy, necessitating the implementation of combination regimens to achieve enhanced antitumor efficacy. Optimizing drug administration sequences is pivotal to harnessing synergistic effects and achieving superadditive therapeutic outcomes (1 + 1 > 2). Erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, dynamically reprograms apoptotic pathways, sensitizing tumor cells to subsequent DNA-damaging agents like doxorubicin within a defined temporal window, thereby augmenting chemotherapy efficacy. To advance this strategy toward clinical relevance, we developed a pH-responsive nanoplatform (PEDHNPs) for sequential control of drug delivery. This system integrates a dual acid-responsive polymeric architecture comprising a hydrazone-linked polycarbonate conjugated with doxorubicin as a prodrug and a ketal-based polycarbonate enabling temporal regulation of drug release. Erlotinib is encapsulated within the hydrophobic core during micelle self-assembly. In the acidic tumor microenvironment, PEDHNPs rapidly liberate erlotinib via ketal hydrolysis, followed by sustained doxorubicin release through hydrazone cleavage. This orchestrated delivery enhances EGFR inhibition and activates caspase-8-mediated apoptosis, potentiating doxorubicin’s antitumor effect. In vitro experiments showed that at a doxorubicin concentration of 80 µg/mL, PEDHNPs achieved a proliferation inhibition rate of 71.54 ± 0.42 % in A549 cells, which was significantly higher than that of the monotherapy groups (Erlotinib: 31.48 ± 0.19 %; Doxorubicin: 63.18 ± 1.04 %) and the control group with amide bond conjugation (54.21 ± 1.13 %). In the NSCLC mouse model, treatment with PEDHNPs resulted in a 95.1 % reduction in tumor volume compared to the control PBS group. These offer a promising paradigm for achieving precise cancer therapy through sequential drug delivery.
Statement of significance
Tumor heterogeneity compromises the efficacy of monotherapies, necessitating rationally designed combination regimens. Optimizing drug administration sequences is critical for harnessing synergistic interactions and achieving superadditive therapeutic outcomes (1 + 1 > 2). Here, a pH-responsive polycarbonate-based nanoparticle (PEDHNP) incorporating hydrazone and ketal linkages was designed for sequential co-delivery of erlotinib and doxorubicin. This sequence-controlled release strategy achieves early EGFR inhibition followed by activation of caspase-8–mediated apoptosis, thereby potentiating the antitumor activity of doxorubicin. In vitro, PEDHNPs exhibited superior antiproliferative and proapoptotic effects compared with monotherapies, single-drug nanoparticles, and amide-linked nanoparticle controls. In vivo, PEDHNPs achieved marked tumor growth suppression in a non-small cell lung cancer model, establishing a versatile platform for precision oncology via sequential drug delivery.
ph响应聚碳酸酯纳米平台使序贯药物释放增强凋亡级联协同作用在非小细胞肺癌治疗中。
肿瘤的异质性给有效的癌症治疗带来了巨大的挑战,需要实施联合治疗方案来提高抗肿瘤疗效。优化给药顺序是利用协同效应和实现超加性治疗结果(1+1 bb0 2)的关键。厄洛替尼是一种表皮生长因子受体(EGFR)抑制剂,可动态重编程凋亡通路,使肿瘤细胞对随后的dna损伤药物(如阿霉素)敏感,从而提高化疗效果。为了将这一策略推向临床应用,我们开发了一种ph响应纳米平台(PEDHNPs),用于药物递送的时空控制。该系统集成了一种双酸反应聚合物结构,包括一种腙连接的聚碳酸酯与阿霉素作为前药偶联,以及一种基于金酮的聚碳酸酯,可以对药物释放进行时间调节。厄洛替尼在胶束自组装过程中被封装在疏水核心内。在酸性肿瘤微环境中,PEDHNPs通过酮酮水解快速释放厄洛替尼,随后通过腙裂解持续释放阿霉素。这种精心安排的递送增强了EGFR抑制,激活了caspase-8介导的细胞凋亡,增强了阿霉素的抗肿瘤作用。体外实验表明,PEDHNPs对A549细胞的增殖抑制率为71.54±1.13%,明显高于单药治疗组(埃洛替尼组:31.48±0.36%;阿霉素组:63.18±1.95%)和酰胺键偶联对照组(54.22±2.14%)。在NSCLC小鼠模型中,与对照PBS组相比,PEDHNPs治疗导致肿瘤体积减少95.1%。这为通过顺序给药实现精确的癌症治疗提供了一个有希望的范例。意义声明:肿瘤异质性影响单药治疗的疗效,需要合理设计联合治疗方案。优化给药顺序对于利用协同相互作用和实现超加性治疗结果(1+1 bb0 2)至关重要。本文设计了一种ph响应型聚碳酸酯纳米颗粒(PEDHNP),结合腙和酮键,用于厄洛替尼和阿霉素的顺序共递送。这种序列控制释放策略实现了早期EGFR抑制,随后激活caspase-8介导的细胞凋亡,从而增强了阿霉素的抗肿瘤活性。在体外,与单一疗法、单药纳米颗粒和酰胺连接纳米颗粒对照相比,PEDHNPs表现出优越的抗增殖和促凋亡作用。在体内,PEDHNPs在非小细胞肺癌模型中实现了显著的肿瘤生长抑制,通过序贯给药建立了精准肿瘤的多功能平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


