Diagnostic accuracy of a host response test in suspected community-Acquired pneumonia during the COVID-19 era
IF 4.3
2区 医学
Q1 INFECTIOUS DISEASES
引用次数: 0
Abstract
Community-acquired pneumonia [CAP] is a leading cause of morbidity and mortality, often complicated by diagnostic uncertainty and antibiotic overuse. This study evaluated the MeMed BV® host-response test in adults with suspected CAP, using clinical management and molecular detection as reference standards.
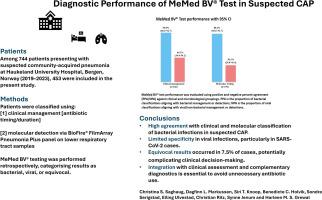
Among 744 patients presenting with suspected CAP at Haukeland University Hospital, Bergen, Norway (2019-2023), across three prospective studies, 453 were included in the present study. Patients were classified using: [1] clinical management [antibiotic timing/duration], and [2] molecular detection via BioFire® FilmArray Pneumonia Plus panel on lower respiratory tract samples. MeMed BV® testing was performed retrospectively on stored serum/plasma, categorising results as bacterial, viral, or equivocal. Healthy controls [n = 20] were also tested.
Among 442 patients classified by clinical management, the MeMed BV® test demonstrated a positive percent agreement [PPA] of 90.0% (95% CI: 86.4-92.7) and a negative percent agreement [NPA] was 44.1% (95% CI: 34.4-54.2). In 370 patients classified by molecular testing, PPA was 88.2% (95% CI: 83.6-91.7) and NPA was 30.1% (95% CI: 20.8-41.4. Equivocal results occurred in 7.5%. The test agreed with clinical management in 96.1% of cases with no detected pathogen. None of the healthy controls had bacterial scores.
Conclusion
MeMed BV® demonstrated high sensitivity in identifying bacterial infections but limited specificity in viral infections, notably SARS-CoV-2. It may aid early triage when combined with clinical and microbiological data.
Trial registration
ClinicalTrials.gov NCT04660084
COVID-19时期宿主反应试验对疑似社区获得性肺炎的诊断准确性
社区获得性肺炎(CAP)是发病率和死亡率的主要原因,通常因诊断不确定和抗生素过度使用而复杂化。本研究以临床管理和分子检测为参考标准,对疑似CAP成人患者的MeMed BV®宿主反应试验进行评估。在挪威卑尔根豪克兰大学医院(Haukeland University Hospital, Bergen, Norway)的744名疑似CAP患者(2019-2023年)中,在三项前瞻性研究中,453名患者被纳入本研究。对患者进行分类:[1]临床管理(抗生素时间/持续时间)和[2]分子检测,通过BioFire®FilmArray Pneumonia Plus面板对下呼吸道样本进行检测。对储存的血清/血浆进行回顾性MeMed BV®检测,将结果分类为细菌、病毒或模棱两可。健康对照[n=20]也进行了检测。在按临床管理分类的442例患者中,MeMed BV®检测显示阳性一致性[PPA]为90.0% (95% CI: 86.4-92.7),阴性一致性[NPA]为44.1% (95% CI: 34.4-54.2)。在370例分子检测分类患者中,PPA为88.2% (95% CI: 83.6-91.7), NPA为30.1% (95% CI: 20.8-41.4)。7.5%出现模棱两可的结果。96.1%未检出病原菌的病例符合临床管理。健康对照组没有细菌得分。结论:MeMed BV®对细菌感染具有较高的敏感性,但对病毒感染的特异性有限,尤其是SARS-CoV-2。当与临床和微生物数据相结合时,它可能有助于早期分类。试验注册:ClinicalTrials.gov NCT04660084。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
18.90
自引率
2.40%
发文量
1020
审稿时长
30 days
期刊介绍:
International Journal of Infectious Diseases (IJID)
Publisher: International Society for Infectious Diseases
Publication Frequency: Monthly
Type: Peer-reviewed, Open Access
Scope:
Publishes original clinical and laboratory-based research.
Reports clinical trials, reviews, and some case reports.
Focuses on epidemiology, clinical diagnosis, treatment, and control of infectious diseases.
Emphasizes diseases common in under-resourced countries.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


