In vitro/in vivo evaluation of 3D bioprinted silk fibroin hydrogels for IBD: A dual mesalazine and TNF-α siRNA approach
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Inflammatory bowel disease (IBD) is a chronic, relapsing disease that poses significant challenges in treatment. This study aimed to develop silk fibroin-based mesalazine and chitosan:TNF-α siRNA polyplex-loaded, 3D bioprinted hydrogels for the oral treatment of IBD. For this purpose, bioink formulations composed of silk fibroin, hyaluronic acid, and sodium alginate were optimized. A chitosan:TNF-α siRNA polyplex was also formulated at a 40:1 chitosan-to-siRNA ratio. Hydrogel formulations were fabricated using 3D bioprinting and characterized in terms of compatibility, thermal stability, swelling behavior, degradation, mechanical properties, and mucoadhesion to both healthy and IBD-induced colon tissues. The optimized oral hydrogel (H-M-P12) demonstrated a swelling index of 366±67 % and underwent 31.2 % degradation after 24 h in vitro. Mesalazine and TNF-α siRNA exhibited a sustained release profile from the hydrogels. Cytotoxicity studies confirmed the biocompatibility of the hydrogels and a TNF-α gene silencing efficiency of 46.53 % was obtained. In vivo studies in a Balb/c mouse model of IBD revealed significant improvements in physiological parameters, macroscopic and microscopic morphology, and biochemical markers following treatment with the developed hydrogels. These findings suggest that silk fibroin-based hydrogels incorporating mesalazine and chitosan/TNF-α siRNA polyplex, produced via 3D bioprinting, hold promise as an effective therapeutic approach for IBD.
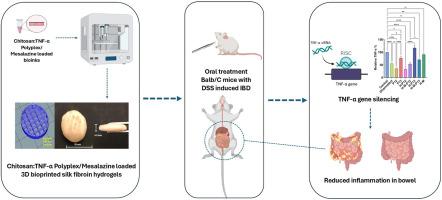
体外/体内评价生物3D打印丝素水凝胶治疗IBD:双美沙拉嗪和TNF-α siRNA方法
炎症性肠病(IBD)是一种慢性、复发性疾病,对治疗提出了重大挑战。本研究旨在开发基于丝素蛋白的美沙拉嗪和壳聚糖、负载TNF-α siRNA的3D生物打印水凝胶用于口服治疗IBD。为此,对丝素蛋白、透明质酸和海藻酸钠组成的生物墨水配方进行了优化。壳聚糖:TNF-α siRNA复合物也以40:1的壳聚糖与siRNA比例配制。利用生物3D打印技术制备水凝胶配方,并对其相容性、热稳定性、肿胀性、降解性、机械性能以及与健康和ibd诱导的结肠组织的黏附性进行了表征。优化后的口服水凝胶(H-M-P12)体外24 h肿胀指数为366±67%,降解率为31.2%。美沙拉嗪和TNF-α siRNA从水凝胶中缓释。细胞毒性研究证实了水凝胶的生物相容性,获得了46.53%的TNF-α基因沉默效率。在Balb/c小鼠IBD模型的体内研究显示,经开发的水凝胶处理后,生理参数、宏观和微观形态以及生化指标均有显著改善。这些发现表明,通过生物3D打印生产的含有美沙拉嗪和壳聚糖/TNF-α siRNA复合物的丝素蛋白水凝胶有望成为IBD的有效治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


