Rbm8a deficiency causes hematopoietic defects by modulating Wnt/PCP signaling
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Thrombocytopenia-Absent Radius (TAR) syndrome is a rare congenital condition with reduced platelets, forelimb anomalies, and variable heart and kidney defects. TAR syndrome is caused by mutations in RBM8A/Y14, a component of the exon junction complex. How perturbing a general mRNA-processing factor causes the selective TAR Syndrome phenotypes remains unknown. Here, we connect zebrafish rbm8a perturbation to early hematopoietic defects via attenuated non-canonical Wnt/Planar Cell Polarity (PCP) signaling. In hypomorphic rbm8a zebrafish, we observe a reduction of cd41-positive thrombocytes. rbm8a-mutant zebrafish accumulate mRNAs with retained introns, including non-canonical Wnt/PCP pathway components resulting in convergent extension defects. We found that reduced rbm8a function interacts with perturbations in non-canonical Wnt/PCP pathway genes wnt5b, wnt11f2, fzd7a, and vangl2, impairing the architecture of the lateral plate mesoderm (LPM) that forms hematopoietic, cardiovascular, kidney, and forelimb skeleton progenitors. Both mutants for rbm8a and for the PCP gene vangl2 feature impaired expression of early hematopoietic/endothelial genes runx1 and gfi1aa. Together, our data propose aberrant LPM patterning and hematopoietic defects as consequence of attenuated non-canonical Wnt/PCP signaling upon reduced rbm8a function.
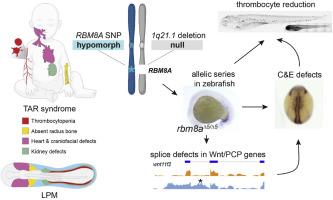
Rbm8a缺乏通过调节Wnt/PCP信号导致造血缺陷。
血小板减少-桡骨缺失综合征(TAR)是一种罕见的先天性疾病,伴有血小板减少、前肢异常以及可变的心脏和肾脏缺陷。TAR综合征是由RBM8A/Y14突变引起的,RBM8A/Y14是外显子连接复合体的一个组成部分。干扰一般mrna加工因子如何导致选择性TAR综合征表型仍然未知。在这里,我们通过减弱的非规范Wnt/平面细胞极性(PCP)信号将斑马鱼rbm8a扰动与早期造血缺陷联系起来。在半胚rbm8a斑马鱼中,我们观察到cd41阳性血小板减少。rbm8a突变斑马鱼积累带有保留内含子的mrna,包括非规范的Wnt/PCP途径成分,导致趋同扩展缺陷。我们发现rbm8a功能的降低与非典型Wnt/PCP通路基因wnt5b、wnt11f2、fzd7a和vangl2的扰动相互作用,损害形成造血、心血管、肾脏和前肢骨骼祖细胞的侧板中胚层(LPM)的结构。rbm8a和PCP基因vangl2的两个突变体都表现出早期造血/内皮基因runx1和gfi1aa的表达受损。总之,我们的数据表明,rbm8a功能降低后,非规范Wnt/PCP信号减弱,导致LPM模式异常和造血缺陷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


