Electrode configuration engineering in a photocatalytic fuel cell for enhanced dye removal and electricity generation
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Photocatalytic fuel cells (PFCs) enable simultaneous wastewater treatment and energy recovery. This study systematically investigated the spatial configuration of electrodes—position, orientation, and distance—in a multi-electrode PFC. Anodic TiO2 nanotube arrays (TNAs/Ti) and cathodic CuO/Cu, both synthesized via anodization, were arranged in six different configurations (S1–S6) and evaluated for acid orange 7 (AO7) removal and electricity generation. The results demonstrated that the heterogeneous configurations (S1 and S3) induced reverse currents from stronger to weaker anodes, which accelerated electron-hole recombination and increased the internal resistance to 600 Ω. In contrast, the homogeneous configuration (S5) minimized the average anode-cathode distance to 5.8 cm, suppressed reverse currents, and enhanced charge separation. This optimized configuration achieved 94.1 % decolorization over 16 h with a pseudo-first-order rate constant of 0.182 h−1, a maximum power density of 11.6 mW m−2, a short-circuit current density of 131 mA m−2, and an internal resistance of 300 Ω. Performance was further enhanced under optimal conditions, including 50 mg L−1 AO7, 0.1 M Na2SO4 electrolyte, pH 5.0, and 1000 Ω external resistance. Radical scavenging tests identified sulfate (SO4·−), superoxide (Ȯ2−), and hydroxyl (ȮH) radicals as primary reactive species with contributions of 35.4 %, 35.0 % and 24.9 %, respectively. Mineralization analyses confirmed 66.7 % COD and 50.5 % TOC removal, with the average oxidation state increasing from −1.4 to 0.4. The system maintained an efficiency of 94.1 % after 20 cycles, with negligible corrosion. These findings indicate that electrode configuration engineering is a crucial strategy for enhancing PFC efficiency.
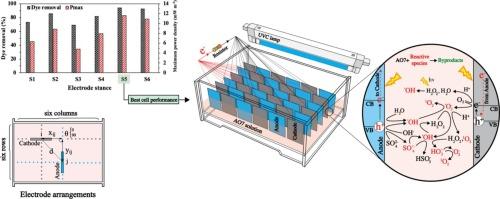
用于增强染料去除和发电的光催化燃料电池的电极配置工程
光催化燃料电池(pfc)可以同时进行废水处理和能量回收。本研究系统地研究了多电极pfc中电极的位置、取向和距离的空间构型。通过阳极氧化合成的阳极TiO2纳米管阵列(tna /Ti)和阴极CuO/Cu排列成六种不同的构型(S1-S6),并对酸性橙7 (AO7)的去除和发电进行了评价。结果表明,非均质结构(S1和S3)诱导了从强阳极向弱阳极的反向电流,加速了电子-空穴复合,使内阻增加到600 Ω。相比之下,均匀结构(S5)将平均阳极-阴极距离减小到5.8 cm,抑制了反向电流,并增强了电荷分离。优化后的结构在16 h内脱色率达到94.1%,伪一阶速率常数为0.182 h−1,最大功率密度为11.6 mW m−2,短路电流密度为131 mA m−2,内阻为300 Ω。在最佳条件下,包括50 mg L−1 AO7, 0.1 M Na2SO4电解质,pH 5.0和1000 Ω外部电阻,性能进一步提高。自由基清除试验发现,硫酸盐(SO4·−)、超氧化物(Ȯ2−)和羟基(ȮH)自由基是主要的活性物质,贡献分别为35.4%、35.0%和24.9%。矿化分析证实COD去除率为66.7%,TOC去除率为50.5%,平均氧化态从−1.4增加到0.4。经过20次循环后,该系统的效率保持在94.1%,腐蚀可以忽略不计。这些发现表明,电极配置工程是提高PFC效率的关键策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


