Localized corrosion and degradation of PCB-ImAg in sulfur-containing environments: a comparative study with PCB-Cu
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
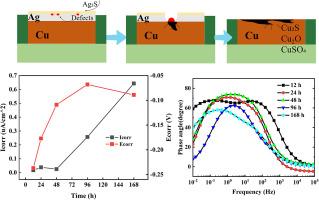
This study investigates the dynamic electrochemical corrosion behavior of copper-clad (PCB-Cu) and immersion silver (PCB-ImAg) circuit boards in a 0.1 mol/L NaHSO₃ solution. PCB-Cu exhibits continuous and rapid corrosion, characterized by the formation of porous copper sulfides and oxides, with its corrosion rate steadily increasing over time. In contrast, PCB-ImAg undergoes a complex, multi-stage degradation. Initially, a partially protective Ag₂S layer forms, showing an early positive shift in Ecorr and a temporary reduction in Icorr, indicating film maturation. However, inherent microdefects in the immersion silver coating act as initiation sites, driving accelerated localized galvanic corrosion between silver and the exposed copper substrate. This leads to a progressive increase in overall corrosion rate, culminating in a distinct negative shift in Ecorr and a drastic surge in Icorr at 168 h, signifying ultimate protective breakdown and accelerated corrosion. EIS, Raman, and XPS analyses confirm a dynamic film evolution, severe structural degradation, and the dominance of Cu₂S as a late-stage corrosion product, contributing to mechanical delamination. This work provides critical insights into the interplay of microstructural defects, film dynamics, and galvanic coupling governing PCB reliability in sulfur-rich environments.
pcb - image在含硫环境中的局部腐蚀和降解:与PCB-Cu的比较研究
本研究研究了包铜(PCB-Cu)和浸银(PCB-ImAg)电路板在0.1 mol/L NaHSO₃溶液中的动态电化学腐蚀行为。PCB-Cu具有连续快速腐蚀的特点,主要表现为多孔硫化铜和氧化物的形成,并且随着时间的推移,其腐蚀速率稳步增加。相比之下,pcb - image经历了一个复杂的、多阶段的降解。一开始,部分保护性的Ag₂S层形成,Ecorr早期正转移,Icorr暂时降低,表明膜成熟。然而,浸没银涂层中固有的微缺陷作为起始点,加速了银与暴露的铜衬底之间的局部电偶腐蚀。这导致整体腐蚀速率逐渐增加,最终导致Ecorr出现明显的负变化,Icorr急剧上升至168 h,这意味着最终的保护击穿和加速腐蚀。EIS, Raman和XPS分析证实了动态膜演化,严重的结构降解,Cu₂S作为后期腐蚀产物占主导地位,导致机械分层。这项工作为微结构缺陷、薄膜动力学和在富硫环境中控制PCB可靠性的电偶耦合的相互作用提供了重要的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


