Chondroitin Sulphate-based hydrogels loaded with folic acid-functionalized nanoparticles to target colorectal cancer
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-03
DOI:10.1016/j.jddst.2025.107479
引用次数: 0
Abstract
Colorectal cancer (CRC) patients continue to be a major cause of cancer-associated morbidity and mortality worldwide. Traditional chemotherapies are least effective due to low bioavailability of drugs at tumor locations, off-target systemic toxicity, and poor oral delivery of hydrophobic drugs. This research describes the first smart nanoparticles-loaded hydrogel system (SNHS) capable of targeted, sustained oral delivery of chemotherapeutics. The system combines folic acid-conjugated nanoparticles for targeted delivery to tumors. Along with pH-sensitive polymeric hydrogels capable of controlled release in the gastrointestinal tract. Comprehensive physicochemical analysis, such as X-ray diffraction, FTIR, SEM, thermal analysis, and dynamic light scattering, assured nanoparticles' stability and ligand conjugation. In-vitro studies proved effective drug loading, entrapment, and sustained release profile up to 70 h in simulated gastric and intestinal fluids. Cellular uptake and cytotoxicity tests on folate receptor-positive (HT-29, HeLa) and FR-negative (A-549) cell lines support receptor-mediated nanoparticle internalization and selective toxicity. The MTT assay on non-cancerous CCD841 cells showed the safety of the developed formulations for non-cancerous healthy cells. The in vivo assessment in the Ht-29 xenograft model of colorectal cancer identified significant inhibition of tumor growth and enhanced survival, tracked by IVIS imaging. The synergistic benefits of targeted nanoparticle delivery and hydrogel-mediated sustained release highlight the potential of SNHS as a viable oral therapeutic platform for CRC. This strategy overcomes major limitations of existing chemotherapy by increasing drug bioavailability, specificity, and patient compliance.
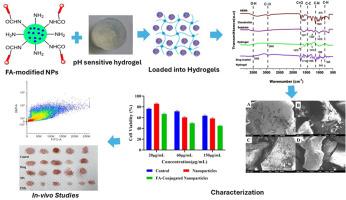
以硫酸软骨素为基础的水凝胶装载叶酸功能化纳米颗粒靶向结直肠癌
结直肠癌(CRC)患者仍然是世界范围内癌症相关发病率和死亡率的主要原因。由于药物在肿瘤部位的生物利用度低、脱靶的全身毒性以及疏水药物的口服递送能力差,传统化疗的效果最差。这项研究描述了第一个智能纳米颗粒负载水凝胶系统(SNHS),能够靶向,持续口服化疗药物。该系统结合了叶酸缀合的纳米颗粒,用于靶向递送肿瘤。与ph敏感的聚合物水凝胶一起能够在胃肠道中控制释放。全面的理化分析,如x射线衍射、红外光谱、扫描电镜、热分析和动态光散射,确保了纳米颗粒的稳定性和配体的共轭性。体外研究证明,在模拟胃液和肠液中,有效的药物装载、包裹和持续释放长达70小时。叶酸受体阳性(HT-29, HeLa)和fr阴性(A-549)细胞系的细胞摄取和细胞毒性试验支持受体介导的纳米颗粒内化和选择性毒性。对非癌性CCD841细胞的MTT试验表明,所研制的制剂对非癌性健康细胞是安全的。结直肠癌Ht-29异种移植模型的体内评估发现,通过IVIS成像可以显著抑制肿瘤生长并提高生存率。靶向纳米颗粒递送和水凝胶介导的缓释的协同效益突出了SNHS作为结直肠癌可行的口服治疗平台的潜力。该策略通过提高药物的生物利用度、特异性和患者依从性,克服了现有化疗的主要局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


