Silencing of METTL14 attenuates experimental intracerebral hemorrhage by suppressing TFRC-mediated ferroptosis
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Ferroptosis is emerging as a pathological mechanism of intracerebral hemorrhage (ICH), and inhibiting ferroptosis contributes to improving prognosis. N6-methyladenosine (m6A) methylation is a common RNA modification that is involved in disease progression. This study aimed to explore the effect of METTL14, a m6A transmethylase, on ferroptosis and the molecular mechanism, and identify its role in ICH progression. PC12 cells were stimulated with hemin to induce injury, and mice were injected with type IV collagenase to generate the ICH model. Ferroptosis was evaluated by detecting Fe2+, reactive oxygen species, and reduced glutathione levels. M6A methylation was assessed by methylated-RNA immunoprecipitation (Me-RIP), RIP, dual-luciferase reporter assay, and actinomycin D treatment. The results showed that hemin induced ferroptosis of PC12 cells. METTL14 was highly expressed in hemin-treated PC12 cells and ICH mice, and knockdown of METTL14 reversed the promotion of ferroptosis induced by hemin. Mechanically, METTL14 knockdown inhibited m6A modification of TFRC and reduced its stability at RNA level, which was recognized by IGF2BP2. Overexpression of TFRC reversed the inhibition of ferroptosis caused by METTL14 silence both in vitro and in vivo. In conclusion, silencing of METTL14 ameliorates ICH by inhibiting ferroptosis through suppressing m6A methylation of TFRC. These findings suggest that targeting m6A methylation and ferroptosis may be a promising strategy for ICH therapy.
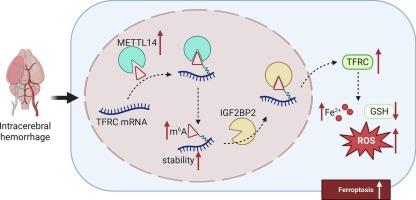
METTL14的沉默通过抑制tfrc介导的铁下垂来减轻实验性脑出血
铁下垂是脑出血的一种病理机制,抑制铁下垂有助于改善预后。n6 -甲基腺苷(m6A)甲基化是一种常见的RNA修饰,与疾病进展有关。本研究旨在探讨m6A转甲基化酶METTL14对铁凋亡的影响及其分子机制,并确定其在脑出血进展中的作用。用hemin刺激PC12细胞诱导损伤,注射IV型胶原酶制备ICH模型。通过检测Fe2+,活性氧和还原性谷胱甘肽水平来评估铁下垂。M6A甲基化通过甲基化rna免疫沉淀(Me-RIP)、RIP、双荧光素酶报告试验和放线菌素D处理进行评估。结果表明,血红素可诱导PC12细胞铁下垂。METTL14在hemin处理的PC12细胞和ICH小鼠中高表达,敲低METTL14可逆转hemin诱导的铁下垂。机械上,METTL14敲低抑制了TFRC的m6A修饰,降低了其在RNA水平上的稳定性,这被IGF2BP2识别。在体外和体内,TFRC的过表达逆转了METTL14沉默引起的铁死亡抑制。综上所述,METTL14的沉默通过抑制TFRC的m6A甲基化来抑制铁凋亡,从而改善ICH。这些发现提示靶向m6A甲基化和铁下垂可能是脑出血治疗的一个有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


