Portal vein-enriched metabolites as intermediate regulators of the gut microbiome in insulin resistance
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Diet and obesity contribute to insulin resistance and type 2 diabetes, in part via the gut microbiome. To explore the role of gut-derived metabolites in this process, we assessed portal/peripheral blood metabolites in mice with different risks of obesity/diabetes, challenged with a high-fat diet (HFD) + antibiotics. In diabetes/obesity-prone C57BL/6J mice, 111 metabolites were portally enriched and 74 were peripherally enriched, many of which differed in metabolic-syndrome-resistant 129S1/129S6 mice. Vancomycin treatment of HFD-fed C57BL/6J mice modified the microbiome and the portal/peripheral ratio of many metabolites, including upregulating tricarboxylic acid (TCA) cycle-related metabolites, like mesaconate, in portal blood. Treatment of isolated hepatocytes with mesaconate, itaconate, or citraconate improved insulin signaling and transcriptionally regulated genes involved in gluconeogenesis, fatty acid oxidation, and lipogenesis in vitro and in vivo. In humans, citraconate levels are inversely correlated with plasma glucose. Thus, portal versus peripheral metabolites play important roles in mediating effects of the microbiome on hepatic metabolism and the pathogenesis of HFD-related insulin resistance.
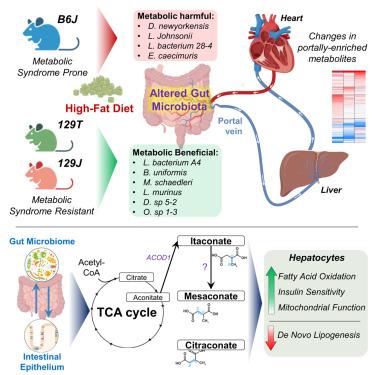
门静脉富集代谢物作为胰岛素抵抗中肠道微生物组的中间调节因子
饮食和肥胖会导致胰岛素抵抗和2型糖尿病,部分原因是肠道微生物群。为了探讨肠道代谢物在这一过程中的作用,我们评估了高脂肪饮食(HFD) +抗生素挑战的不同肥胖/糖尿病风险小鼠的门脉/外周血代谢物。在糖尿病/肥胖易感的C57BL/6J小鼠中,111种代谢物在门静脉富集,74种外周富集,其中许多代谢物在代谢综合征抗性的129S1/129S6小鼠中有所不同。万古霉素治疗hfd喂养的C57BL/6J小鼠改变了微生物组和许多代谢物的门脉/外周比例,包括上调门静脉血液中三羧酸(TCA)循环相关代谢物,如美沙酸酯。用美沙康酸、衣康酸或柠檬酸处理离体肝细胞可改善胰岛素信号传导和参与糖异生、脂肪酸氧化和脂肪生成的转录调节基因。在人类中,柠檬酸盐水平与血浆葡萄糖呈负相关。因此,门静脉与外周代谢物在介导微生物组对肝脏代谢的影响和hfd相关胰岛素抵抗的发病机制中发挥了重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


