MLKL PARylation in the endothelial niche triggers angiocrine necroptosis to evade cancer immunosurveillance and chemotherapy
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
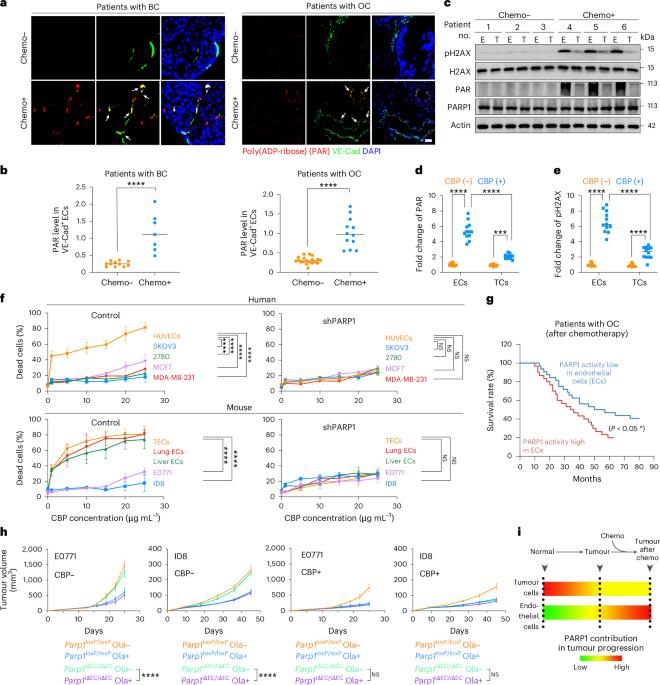
Chemoresistance is the leading cause of cancer-related death. How chemotherapy subjugates the cellular crosstalk in the tumour microenvironment to cause chemoresistance remains to be defined. Here we find chemotherapy enables immunosuppressive SDF1+ endothelial niche to evade immunosurveillance in ovarian and breast cancers. We integrated human patient data and mouse models to show that chemotherapy selectively activates PARP1–SDF1 axis in tumour endothelial cells (ECs). This angiocrine SDF1 interferes with antitumour interplay between CXCL10+ macrophages and CXCR3+CD8+ T cells and promotes tumour progression in ovarian and breast cancers. Proteome-based screening revealed that endothelial PARP1 PARylates MLKL, a key necroptosis effector to upregulate angiocrine SDF1 in ECs. In sum, we identify PARylation-dependent necroptosis in tumour ECs as an important step in subverting the tumour microenvironment to evade immunosurveillance. Yang, Li, Huang, Ji, Luo, Jiang and colleagues report that chemotherapy induces MLKL PARylation and necroptosis in tumour endothelial cells, which in turn affects tumour-associated macrophages and CD8⁺ T cells, promoting immunosuppression and tumorigenesis.
内皮龛中的MLKL PARylation触发血管分泌性坏死性坏死,以逃避癌症免疫监视和化疗
化疗耐药性是癌症相关死亡的主要原因。化疗如何抑制肿瘤微环境中的细胞串扰导致化疗耐药仍有待确定。在这里,我们发现化疗使免疫抑制性SDF1+内皮小生境逃避卵巢癌和乳腺癌的免疫监视。我们综合了人类患者数据和小鼠模型,表明化疗选择性地激活肿瘤内皮细胞(ECs)中的PARP1-SDF1轴。这种血管分泌素SDF1干扰CXCL10+巨噬细胞和CXCR3+CD8+ T细胞之间的抗肿瘤相互作用,并促进卵巢癌和乳腺癌的肿瘤进展。基于蛋白质组学的筛选显示内皮细胞PARP1与MLKL相结合,MLKL是内皮细胞中上调血管分泌SDF1的关键坏死性坏死效应因子。总之,我们发现肿瘤上皮细胞中paryl依赖性坏死下垂是破坏肿瘤微环境以逃避免疫监视的重要步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


