Pseudomonas aeruginosa senses exopolysaccharide trails using type IV pili and adhesins during biofilm formation
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
During early stages of biofilm formation, Pseudomonas aeruginosa (Pa) PAO1 can sense exopolysaccharide (EPS) trails of Psl deposited on a surface by previous Pa cells to detect trajectories of other cells and to orchestrate motility. This sensory signal is transduced into cyclic diGMP second messengers, but no known Psl receptors and adhesins participate in signal transduction. Here, using bacteria-secreted Psl trails, glycopolymer-patterned surfaces, longitudinal cell tracking, second messenger dual reporters and genetic mutations targeting EPS binding and surface twitching, we find that Pa is capable of sensing EPS directly through mutually constitutive interactions between type IV pili (T4P)-powered twitching and specific adhesin–EPS bonds. This unanticipated mechanochemical surveillance of the Pa environment, where T4P pull against cell-body localized adhesins interacting with EPS trails, such as mannose-binding CdrA, generates a hybrid, transitional planktonic-to-biofilm population with elevated cyclic diGMP and elevated cyclic AMP, as well as increased motility capable of following EPS trails. These results show a generalizable mechanism of surface chemosensing through mechanosensitive appendages. Opposing forces generated by exopolysaccharide trail binding versus type IV pilus retraction generate a high cyclic diGMP–high cyclic AMP state in Pseudomonas aeruginosa that promotes social motility.

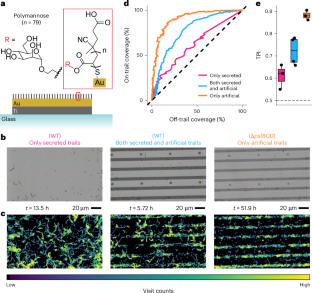
铜绿假单胞菌在生物膜形成过程中利用IV型菌毛和粘附素感知胞外多糖
在生物膜形成的早期阶段,铜绿假单胞菌(Pa) PAO1可以感知先前Pa细胞沉积在表面的Psl的胞外多糖(EPS)轨迹,从而检测其他细胞的运动轨迹并协调运动。这种感觉信号被转导成循环diGMP第二信使,但没有已知的Psl受体和粘附素参与信号转导。在这里,利用细菌分泌的Psl轨迹、糖共聚物图案表面、纵向细胞跟踪、第二信使双报告和针对EPS结合和表面抽搐的基因突变,我们发现Pa能够通过IV型菌毛(T4P)驱动的抽搐和特异性黏附- EPS键之间的相互构成相互作用直接感知EPS。这种意想不到的Pa环境的机械化学监测,其中T4P拉力对抗与EPS路径相互作用的细胞体局部粘附素,如甘露糖结合CdrA,产生杂交,浮游生物向生物膜过渡的群体,具有升高的环状diGMP和升高的环状AMP,以及能够跟随EPS路径的运动性增加。这些结果显示了一种通过机械敏感附属物进行表面化学感应的可推广机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


