Thiol-epoxy ‘click’ reaction in polymer synthesis
IF 26.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
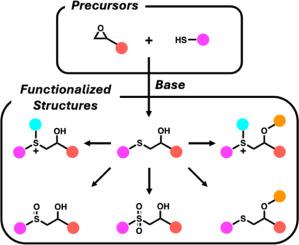
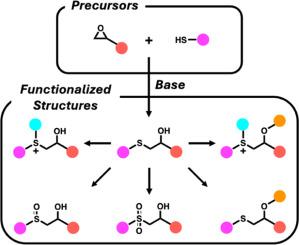
The base-catalyzed ring-opening reaction of epoxides by thiol nucleophiles, commonly known as the thiol-epoxy ‘click’ reaction, is a versatile method for forming thioether bonds. This review offers mechanistic insights into the reaction and explores its applications in polymer synthesis. The discussion also includes post-polymerization modifications of thioether linkages into sulfoxides, sulfones, and cationic sulfonium salts, as well as esterification of the secondary hydroxyl groups generated by the ‘click’ reaction. Additional topics include scalability, chemoselectivity, regioselectivity, and the formation of disulfide defects. Practical recommendations are provided for optimizing reaction conditions and minimizing side reactions. Finally, future directions are proposed to further expand the utility of this reaction in polymer chemistry.
聚合物合成中的硫醇-环氧“点击”反应
巯基亲核试剂催化环氧化合物开环反应,通常称为巯基-环氧“咔嗒”反应,是形成硫醚键的一种通用方法。本文综述了该反应的机理,并探讨了其在聚合物合成中的应用。讨论还包括聚合后修饰的硫醚连接到亚砜,砜和阳离子磺盐,以及二级羟基的酯化产生的“点击”反应。其他主题包括可伸缩性、化学选择性、区域选择性和二硫化缺陷的形成。提出了优化反应条件和减少副反应的实用建议。最后,提出了进一步扩大该反应在高分子化学中的应用的发展方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Progress in Polymer Science
化学-高分子科学
CiteScore
48.70
自引率
1.10%
发文量
54
审稿时长
38 days
期刊介绍:
Progress in Polymer Science is a journal that publishes state-of-the-art overview articles in the field of polymer science and engineering. These articles are written by internationally recognized authorities in the discipline, making it a valuable resource for staying up-to-date with the latest developments in this rapidly growing field.
The journal serves as a link between original articles, innovations published in patents, and the most current knowledge of technology. It covers a wide range of topics within the traditional fields of polymer science, including chemistry, physics, and engineering involving polymers. Additionally, it explores interdisciplinary developing fields such as functional and specialty polymers, biomaterials, polymers in drug delivery, polymers in electronic applications, composites, conducting polymers, liquid crystalline materials, and the interphases between polymers and ceramics. The journal also highlights new fabrication techniques that are making significant contributions to the field.
The subject areas covered by Progress in Polymer Science include biomaterials, materials chemistry, organic chemistry, polymers and plastics, surfaces, coatings and films, and nanotechnology. The journal is indexed and abstracted in various databases, including Materials Science Citation Index, Chemical Abstracts, Engineering Index, Current Contents, FIZ Karlsruhe, Scopus, and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


