Innovative engineering approaches to model host-microbiome interactions in vitro
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The human microbiome plays a critical role in health and disease. Disruptions in microbiota composition or function have been implicated not only as markers but also as drivers of diverse pathologies, creating opportunities for targeted microbiome interventions. Advancing these therapies requires experimental models that can unravel the complex, bidirectional interactions between human tissue and microbial communities. This scoping review examines emerging engineering approaches to design in vitro platforms that successfully integrate host and microbial components to model these interactions. Compared to traditional in vitro and in vivo approaches, these advanced microphysiological systems offer greater experimental control, human-specific biology, and reduced cost and ethical concerns. Here, we identify key challenges in the creation of these in vitro models and innovative solutions to address them by leveraging microfluidics, biomaterials, and organoid technologies, among others. These strategies have enabled the development of co-culture systems that replicate critical features of host-microbiome interfaces, including mucosal barriers, oxygen and pH gradients, mechanical stimuli, and host cell diversity. We also describe how these physiologically relevant models are uncovering new insights into epithelial-microbiota crosstalk, immune modulation by commensal microbes, and systemic effects of microbiota and their metabolites across multiple body sites. We conclude by discussing opportunities to expand these systems in scale, complexity, and clinical relevance. As these models continue to evolve, they hold the potential to transform our ability to mechanistically probe microbiome interactions, personalize therapeutic strategies, and accelerate the translation of microbiome science into clinical practice.
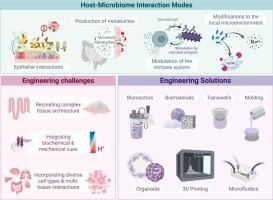
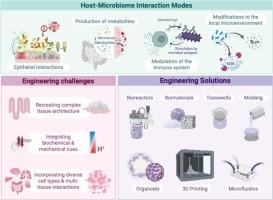
体外模拟宿主-微生物相互作用的创新工程方法
人体微生物组在健康和疾病中起着至关重要的作用。微生物群组成或功能的破坏不仅是标志物,也是多种病理的驱动因素,为靶向微生物组干预创造了机会。推进这些疗法需要实验模型来解开人体组织和微生物群落之间复杂的双向相互作用。这篇综述研究了新兴的工程方法来设计成功整合宿主和微生物成分来模拟这些相互作用的体外平台。与传统的体外和体内方法相比,这些先进的微生理系统提供了更好的实验控制,人类特异性生物学,降低了成本和伦理问题。在这里,我们确定了创建这些体外模型的关键挑战,并通过利用微流体、生物材料和类器官技术等创新解决方案来解决这些问题。这些策略使得共培养系统的发展能够复制宿主-微生物组界面的关键特征,包括粘膜屏障、氧和pH梯度、机械刺激和宿主细胞多样性。我们还描述了这些生理学相关模型如何揭示上皮-微生物群串扰、共生微生物的免疫调节以及微生物群及其代谢物在多个身体部位的全身作用的新见解。最后,我们讨论了在规模、复杂性和临床相关性方面扩大这些系统的机会。随着这些模型的不断发展,它们有可能改变我们机械地探测微生物组相互作用的能力,个性化治疗策略,并加速微生物组科学向临床实践的转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


