“ TPGS-Functionalized Nanocarriers with Improved Flavonoid Oral Bioavailability and Therapeutic Action: Pharmacokinetic and Mechanistic Insights in Diabetes-Induced Retinopathy ”
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-01
DOI:10.1016/j.ejpb.2025.114851
引用次数: 0
Abstract
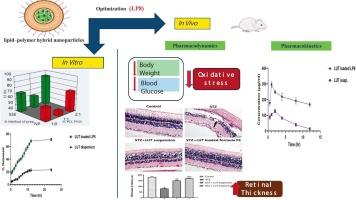
Diabetes mellitus is a metabolic disorder with escalating prevalence. It’s a chief cause of microvascular complications, notably diabetic retinopathy (DR), which can predispose to permanent vision loss. Inflammation and oxidative stress are pivotal contributors to the progression of DR. Luteolin (LUT), a naturally occurring flavonoid, possesses strong anti-inflammatory and antioxidant properties, offering therapeutic potential against DR. However, its clinical use is impeded by its restricted intestinal permeability and poor water solubility. In this study, twelve lipid–polymer hybrid nanoparticles (LPNPs) were developed using a 1322 complete factorial design to explore the impact of three formulation variables: poly(ε-caprolactone) (PCL): d-alpha-Tocopheryl polyethylene glycol 1,000 succinate (TPGS) ratio (A), preparation technique (B) and phospholipid (PL) content (C). The optimized formulation (LP8), composed of 50 mg of phospholipid, PC: TPGS (2:1) and prepared via the emulsion solvent evaporation method, demonstrated a high zeta potential (−34.9 ± 5.2 mV), a small particle size (215.3 ± 10.3 nm), and a high entrapment efficiency (93.1 ± 2.4 %). Compared to LUT suspension, LP8 demonstrated prolonged in vitro drug release and markedly enhanced ex vivo intestinal permeability. In streptozotocin (STZ)-induced diabetes, LP8 resulted in significant reduction in hyperglycemia, retinal inflammation, oxidative stress and angiogenesis while preserving retinal structure. Moreover, LP8 significantly downregulated the expression of MDA, IL-1β, NLRP3, ASC, and VEGF while upregulated GSH and Nrf2 levels in the retina. Additionally, pharmacokinetic study confirmed a substantial improvement in oral bioavailability of LUT-loaded LP8 compared to LUT-suspension. These findings proposed that the optimized LUT-loaded LPNPs represent a potential oral nanoplatform for the effective management of DR.
“具有改善类黄酮口服生物利用度和治疗作用的tpgs功能化纳米载体:糖尿病诱导视网膜病变的药代动力学和机制见解”。
糖尿病是一种发病率不断上升的代谢性疾病。它是微血管并发症的主要原因,尤其是糖尿病视网膜病变(DR),这可能导致永久性视力丧失。炎症和氧化应激是dr进展的关键因素。木犀草素(Luteolin, LUT)是一种天然存在的类黄酮,具有很强的抗炎和抗氧化特性,具有治疗dr的潜力。然而,它的临床应用受到肠道渗透性和水溶性的限制。本研究采用1322全因子设计,制备了12种脂质-聚合物杂化纳米颗粒(lnps),探讨了三个配方变量:聚(ε-己内酯)(PCL): d- α -生育酚基聚乙二醇1000丁二酸酯(TPGS)配比(a)、制备工艺(B)和磷脂(PL)含量(C)对lnps的影响。优化的配方(LP8),由50 毫克的磷脂,PC: tpg(2:1),准备通过乳化溶剂蒸发法,证明高电动电势(-34.9 ±5.2 mV),一个小粒径(215.3 ±10.3 海里),和一个诱捕效率高(93.1 ±2.4 %)。与LUT混悬液相比,LP8延长了体外药物释放时间,并显著提高了体外肠道通透性。在链脲佐菌素(STZ)诱导的糖尿病中,LP8显著降低高血糖、视网膜炎症、氧化应激和血管生成,同时保留视网膜结构。此外,LP8显著下调MDA、IL-1β、NLRP3、ASC和VEGF的表达,上调视网膜中GSH和Nrf2的水平。此外,药代动力学研究证实,与lut悬浮液相比,lut负载LP8的口服生物利用度有显著提高。这些发现表明,优化的lut负载的lnps代表了有效治疗DR的潜在口服纳米平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


