Whole-brain mapping of afferent and efferent connections of lateral hypothalamic orexinergic neurons in mice
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Orexin (Orx) is a vital peptide neurotransmitter essential for regulating feeding, sleep-wake cycles, and reward-seeking behavior. Orexinergic neurons are predominantly located in the lateral hypothalamus (LH). However, the precise neural connectivity of these neurons across the brain remains insufficiently characterized. In this study, we used cell-specific anterograde and retrograde viral tracers to map the afferent and efferent connections of LH orexinergic neurons. Our results revealed that LH orexinergic neurons received extensive afferent inputs from cortical regions, including the primary motor cortex (M1) and secondary motor cortex (M2), the basal ganglia, including the caudate putamen (CPu), the diencephalon, such as the zona incerta (ZI) and reticular nucleus (Rt), and brainstem nuclei, including the ventral tegmental area (VTA) and locus coeruleus (LC). Simultaneously, the orexinergic neurons projected primarily to the telencephalon, including the bed nucleus of the stria terminalis (BNST) and lateral septum (LS), the diencephalon, including the medial preoptic nucleus (MPA) and lateral preoptic area (LPO), and the brainstem, such as the periaqueductal gray (PAG) and superior colliculus (SC). Among these, BNST, LS, MPA, and PAG were the primary projection targets of LH orexinergic neurons, whereas ZI, VTA, M1, and LC serve as their major input sources. Additionally, significant bidirectional projections were observed between LH orexinergic neurons and regions such as ZI and VTA, suggesting their potential roles in signal integration. These findings provide a detailed framework for the neural connectivity of LH orexinergic neurons, highlighting their potential involvement in mediating complex brain functions and neuropsychiatric disorders.
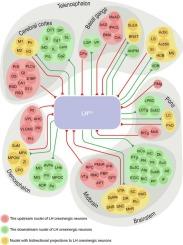
小鼠下丘脑外侧食欲能神经元传入和传出连接的全脑作图。
食欲素(Orx)是一种重要的肽神经递质,对调节进食、睡眠-觉醒周期和寻求奖励行为至关重要。食欲能神经元主要位于外侧下丘脑(LH)。然而,这些神经元在整个大脑中的精确神经连接仍然没有得到充分的表征。在这项研究中,我们使用细胞特异性的逆行和逆行病毒示踪剂来绘制LH食欲能神经元的传入和传出连接。结果表明,LH食欲能神经元接受来自皮层区域的广泛传入输入,包括初级运动皮质区(M1)和次级运动皮质区(M2)、基底节区(CPu)、间脑(incerta区(ZI)和网状核(Rt))以及脑干核(包括腹侧被盖区(VTA)和蓝斑核(LC))。同时,orexinergy神经元主要投射到端脑,包括终纹床核(BNST)和外侧隔核(LS),间脑,包括内侧视前核(MPA)和外侧视前区(LPO),以及脑干,如导水管周围灰质(PAG)和上丘(SC)。其中,BNST、LS、MPA和PAG是LH兴奋能神经元的主要投射靶点,而ZI、VTA、M1和LC是它们的主要输入源。此外,在LH食欲能神经元与ZI和VTA等区域之间观察到显著的双向投射,表明它们可能在信号整合中起作用。这些发现为LH食欲能神经元的神经连接提供了一个详细的框架,强调了它们在介导复杂脑功能和神经精神疾病中的潜在参与。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


