Hydrogen sulfide (H2S) alleviates diabetic myocardial fibrosis by suppressing pyroptosis via inhibiting DNMT3a-mediated Sestrin2 CpG promoter hypermethylation
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
Diabetic cardiomyopathy (DCM) represents an important concern associated with diabetes, inhibiting pyroptosis has shown promising results in alleviating DCM symptoms. The objective of this work is to investigate the role and underlying mechanism of hydrogen sulfide (H2S) in the suppression of pyroptosis in the context of diabetic myocardial fibrosis (MF).
Methods
The effect of H2S on pyroptosis was detected using CCK-8, ELISA, and flow cytometry. The expression of Sestrin2 and its DNA methylation modification, as well as the quantification of pyroptosis-related proteins and the activation of the TLR4/MyD88/NF-κB signaling pathway, were measured using Western blot, ChIP, Immunofluorescence, and methylation-specific quantitative PCR. To establish a type 2 diabetes rat model, a high-fat diet was administered, followed by injection of streptozotocin (HFD/STZ). After five weeks, the rats received H2S treatment for four weeks, either with or without sh-Sestrin2. The effects of H2S treatment on myocardial function, tissue structure, and myocardial cell apoptosis were assessed. Furthermore, the CCK-8 assay was used to detect the cell viability induced by TGF-β. Cellular oxidative stress levels were measured by the ELISA method. Western blotting was applied to determine the protein expression levels of Collagen I, Fibronectin, α-SMA, NLRP3, caspase-1, GSDMD-N, and Sestrin2.
Results
H2S ameliorates HG-induced fibrosis, injury, pyroptosis, and inflammatory response of cardiac fibroblasts (CFs). H2S inhibits Sestrin2 methylation and up-regulates its expression through DNMT3a. H2S ameliorates HG-induced CFs pyroptosis and fibrosis through Sestrin2. Sestrin2 regulates HG-induced CFs pyroptosis and fibrosis through the TLR4/MyD88/NF-κB pathway. The myocardial tissue injury was improved after H2S treatment, and the results of the H2S treatment were reversed after knockdown of Sestrin2. In addition, TGF-β1 can induce an increase in the activity of cardiac fibroblasts and enhance cellular oxidative stress. Meanwhile, it significantly upregulates the expression of Collagen I, Fibronectin, α-SMA, as well as NLRP3, caspase-1, and GSDMD-N, and remarkably downregulates the expression of Sestrin2. However, H2S intervention can reverse the aforementioned phenomena.
Conclusion
H2S can suppress pyroptosis and ameliorate diabetic MF by inhibiting DNMT3a-mediated DNA methylation of Sestrin2 promoter through the TLR4/MyD88/NF-κB pathway.
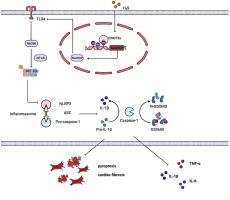
硫化氢(H2S)通过抑制dnmt3a介导的Sestrin2 CpG启动子超甲基化抑制焦亡,减轻糖尿病心肌纤维化。
背景:糖尿病性心肌病(DCM)是与糖尿病相关的一个重要问题,抑制焦亡在缓解DCM症状方面显示出良好的效果。这项工作的目的是研究硫化氢(H2S)在糖尿病心肌纤维化(MF)背景下抑制焦亡的作用和潜在机制。方法:采用CCK-8、ELISA、流式细胞术检测H2S对小鼠焦亡的影响。采用Western blot、ChIP、Immunofluorescence、methyl- specific quantitative PCR检测Sestrin2的表达及其DNA甲基化修饰,以及热降解相关蛋白的定量表达和TLR4/MyD88/NF-κB信号通路的激活情况。采用高脂饮食,注射链脲佐菌素(HFD/STZ)建立2型糖尿病大鼠模型。5周后,大鼠接受H2S治疗4周,有或没有sh-Sestrin2。观察H2S处理对大鼠心肌功能、组织结构及心肌细胞凋亡的影响。CCK-8法检测TGF-β诱导的细胞活力。ELISA法测定细胞氧化应激水平。Western blotting检测ⅰ型胶原蛋白、纤维连接蛋白、α-SMA、NLRP3、caspase-1、GSDMD-N、Sestrin2蛋白的表达水平。结果:H2S可改善hg诱导的心肌成纤维细胞(CFs)纤维化、损伤、焦亡和炎症反应。H2S通过DNMT3a抑制Sestrin2甲基化并上调其表达。H2S通过Sestrin2改善hg诱导的CFs焦亡和纤维化。Sestrin2通过TLR4/MyD88/NF-κB通路调控hg诱导的CFs焦亡和纤维化。H2S处理后心肌组织损伤得到改善,而敲低Sestrin2后H2S处理的结果逆转。此外,TGF-β1可诱导心肌成纤维细胞活性增加,增强细胞氧化应激。同时,显著上调I型胶原蛋白、纤维连接蛋白、α-SMA以及NLRP3、caspase-1、GSDMD-N的表达,显著下调Sestrin2的表达。然而,H2S干预可以扭转上述现象。结论:H2S通过TLR4/MyD88/NF-κB通路抑制dnmt3a介导的Sestrin2启动子DNA甲基化,可抑制糖尿病MF的焦亡,改善糖尿病MF。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


