Quantitative characterization of the interfacial failure of metallic coatings on epoxy substrates in salty atmospheres
IF 2.7
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
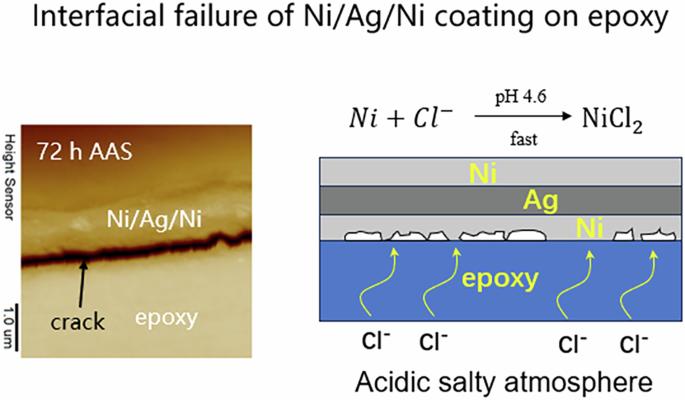
Although a multilayered metallic coatings on epoxy surfaces can enhance material performance, under acidic and salty conditions, such as sweat, the coatings usually delaminate from polymer surfaces. Therefore, we investigated the interfacial failure of a Ni/Ag/Ni coating on an epoxy surface after long-term exposure to salt spray air or artificial sweat containing abundant Cl− ions. Cross-sectional atomic force microscopy and X-ray photoelectron spectroscopy were used to probe the changes in the interfacial morphologies and depth profiles of the chemical composition, respectively, to elucidate the mechanism by which Ni/Ag/Ni trilayered coatings delaminate from epoxy substrates in Cl−-containing atmospheres. The results revealed that Cl− ions penetrated through the epoxy, diffused into the metal–polymer interface, and dissolved the Ni layer at the epoxy surface, detaching the metallic layer from the polymer surface. In response to the failure mechanism mentioned above, we propose that by partially replacing the Ni primer layer with Ni2O3, which is inert in acidic Cl− atmospheres, the Ni2O3/Ni/Ag/Ni coating strongly resists corrosion in salt spray air and possesses good long-term interfacial bonding under acidic Cl− atmospheres. The mechanism of interfacial failure of Ni/Ag/Ni coating on epoxy surface after long-term treatment in air of artificial sweat were evaluated by cross-section AFM and XPS depth profiling. It was revealed that Cl ions penetrate the epoxy substrate, diffuse into metal-polymer interface and dissolve the primer Ni layer, resulting a delamination of metal coating from epoxy substrate. A Ni₂O₃ layer was introduced to construct the Ni₂O₃/Ni/Ag/Ni structure, which exhibits superior interfacial bonding performance in acidic Cl− atmosphere.
含盐环境下环氧基金属涂层界面破坏的定量表征
虽然环氧树脂表面的多层金属涂层可以提高材料的性能,但在酸性和咸的条件下,例如汗水,涂层通常会从聚合物表面分层。因此,我们研究了长期暴露于含有大量Cl−离子的盐雾空气或人工汗液后,环氧树脂表面的Ni/Ag/Ni涂层的界面破坏。利用横截面原子力显微镜和x射线光电子能谱分别探测了界面形貌和化学成分深度分布的变化,以阐明Ni/Ag/Ni三层涂层在含Cl−气氛下从环氧基基体上分层的机理。结果表明:Cl−离子穿过环氧树脂,扩散到金属-聚合物界面,溶解了环氧树脂表面的Ni层,使金属层与聚合物表面分离;针对上述失效机理,我们提出通过用在酸性Cl -气氛中具有惰性的Ni2O3部分取代Ni底漆层,Ni2O3/Ni/Ag/Ni涂层在盐雾空气中具有较强的耐腐蚀性,并且在酸性Cl -气氛中具有良好的长期界面结合。采用横截面原子力显微镜和XPS深度分析方法,研究了在人工汗液空气中长期处理后,环氧树脂表面Ni/Ag/Ni涂层界面失效的机理。结果表明,Cl离子渗透到环氧基材中,扩散到金属-聚合物界面,溶解底漆Ni层,导致环氧基材金属涂层脱层。引入Ni₂O₃层构建Ni₂O₃/Ni/Ag/Ni结构,该结构在酸性Cl−气氛中表现出优异的界面键合性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


