Mechanisms and interventions of Aeromonas hydrophila-induced hepatic injury in Megalobrama amblycephala: Focus on oxidative stress-mediated mitochondrial apoptosis
IF 3.9
1区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
Our previous study demonstrated that Aeromonas hydrophila induces apoptosis and tissue injury in Megalobrama amblycephala. In this study, we explored the specific apoptotic pathways activated by A. hydrophila. Results revealed significant A. hydrophila enrichment in hepatocytes post-infection, which was attenuated by lipid raft inhibitors (methyl-β-cyclodextrin and simvastatin), along with cholesterol depletion. Meanwhile, a significant accumulation of total reactive oxygen species (T-ROS) in hepatocytes was observed, and the increase in T-ROS positive cells was further confirmed by flow cytometry. Additionally, A. hydrophila infection reduced mitochondrial membrane potential (MMP) and increased hepatocyte apoptosis. Western blot analysis revealed decreased mitochondrial cytochrome c and increased cytoplasmic cytochrome c levels after infection, along with significant activation of caspase-9 and caspase-3. To determine whether oxidative stress mediates A. hydrophila induced mitochondrial apoptosis, hepatocytes were pretreated with N-acetylcysteine (NAC) before infection. NAC treatment significantly reduced T-ROS accumulation, alleviated MMP decline and cytochrome c release, and inhibited caspase-induced apoptosis. In vivo experiment similarly demonstrated that NAC significantly attenuated A. hydrophila-induced mitochondrial damage, apoptosis pathway activation, and hepatic tissue vacuolization. In conclusion, this research enhances our understanding of A. hydrophila's pathogenic mechanism and provides a foundation for developing strategies to prevent and treat infections caused by this bacterium in fish.
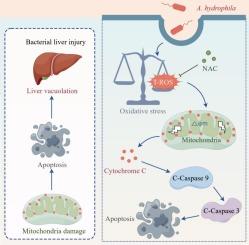
嗜水气单胞菌诱导的双头巨鲷肝损伤机制及干预措施:氧化应激介导的线粒体凋亡
我们前期的研究表明,嗜水气单胞菌可诱导双头巨鲷细胞凋亡和组织损伤。在本研究中,我们探索了嗜水芽孢杆菌激活的特定凋亡途径。结果显示,感染后肝细胞中有显著的嗜水芽胞杆菌富集,脂质筏抑制剂(甲基β-环糊精和辛伐他汀)和胆固醇降低了这种富集。同时,观察到肝细胞中总活性氧(T-ROS)明显积累,流式细胞术进一步证实了T-ROS阳性细胞的增加。此外,嗜水单胞杆菌感染降低了线粒体膜电位(MMP),增加了肝细胞凋亡。Western blot分析显示,感染后线粒体细胞色素c降低,细胞质细胞色素c升高,caspase-9和caspase-3显著激活。为了确定氧化应激是否介导嗜水单胞菌诱导的线粒体凋亡,在感染前用n -乙酰半胱氨酸(NAC)预处理肝细胞。NAC处理显著降低T-ROS积累,缓解MMP下降和细胞色素c释放,抑制caspase诱导的细胞凋亡。体内实验同样表明,NAC可显著减轻嗜水单胞虫诱导的线粒体损伤、凋亡通路激活和肝组织空泡化。总之,本研究增强了我们对嗜水单胞杆菌致病机制的认识,为制定预防和治疗该细菌在鱼类中的感染的策略提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Aquaculture
农林科学-海洋与淡水生物学
CiteScore
8.60
自引率
17.80%
发文量
1246
审稿时长
56 days
期刊介绍:
Aquaculture is an international journal for the exploration, improvement and management of all freshwater and marine food resources. It publishes novel and innovative research of world-wide interest on farming of aquatic organisms, which includes finfish, mollusks, crustaceans and aquatic plants for human consumption. Research on ornamentals is not a focus of the Journal. Aquaculture only publishes papers with a clear relevance to improving aquaculture practices or a potential application.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


