Organocatalytic-assisted synthesis of 1,2,3-triazoles mediated by chitosan beads
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
In this study, we explore the potential of chitosan beads as an organocatalyst for the synthesis of 1,2,3-triazoles. The chitosan beads were prepared using a straightforward coagulation process, yielding beads with a narrow size distribution (2.34 ± 0.21 mm), significant porosity, and high stability under proposed experimental conditions. Under optimized conditions, the chitosan beads (20 mg) exhibited efficient catalytic activity, assisting the synthesis of diverse 1,4,5-trisubstituted-1,2,3-triazoles with yields ranging from 27 % to 97 %. The protocol proved compatible with 1,3-diketones, β-keto-esters, and α-cyano-ketones, while azides bearing electron-withdrawing groups demonstrate enhanced efficiency compared to electron-donating counterparts. Importantly, the bead-like structure of chitosan was shown to be crucial in achieving high yields of 1,2,3-triazoles. This study introduces chitosan as a green and versatile organocatalyst for synthesizing valuable organic compounds like triazoles, offering new insights into its application in sustainable chemistry.
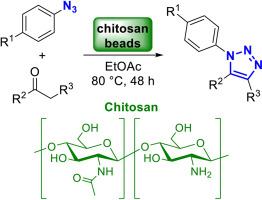
壳聚糖微球介导的有机催化合成1,2,3-三唑
在本研究中,我们探索了壳聚糖珠作为合成1,2,3-三唑的有机催化剂的潜力。采用直接混凝法制备壳聚糖微球,制备出粒径分布窄(2.34±0.21 mm)、孔隙率高、稳定性好的微球。在优化条件下,壳聚糖珠(20 mg)表现出高效的催化活性,可催化合成多种1,4,5-三取代-1,2,3-三唑,收率为27% ~ 97%。该方案被证明与1,3-二酮、β-酮酯和α-氰基酮兼容,而带有吸电子基团的叠氮化合物与供电子基团相比效率更高。重要的是,壳聚糖的珠状结构被证明是实现1,2,3-三唑高产率的关键。本研究介绍了壳聚糖作为一种绿色、多功能的有机催化剂,可用于合成三唑等有价值的有机化合物,为其在可持续化学中的应用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


