Solvent- and pH-responsive fluorescence of an imidazophenanthroline dye and its supramolecular complex with 3,4,5-tris(dodecyloxy)benzoic acid: experimental and theoretical insights
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
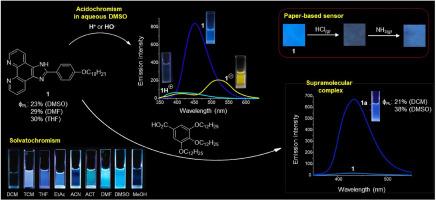
Fluorescent pH-responsive organic structures have attracted great attention due to their roles in biological and environmental fields, as well as in the development of materials science. In this paper, the photochemical and acidochromic properties of 2-(4-(decyloxy)phenyl)-1H-imidazo[4,5-f]phenanthroline (1) were investigated. As verified by fluorescence spectroscopy and naked-eye analysis, dye 1 presents a solvent-dependent emissive behavior, being non-emissive in dichloromethane (DCM) and methanol solutions, but exhibiting moderate to intense fluorescence in all other solvents. While in chloroform (TCM) solution the dye 1 emits in the cyan region, typical blue fluorescence is observed in ethyl acetate (EtAc), acetonitrile (ACN), tetrahydrofuran (THF), acetone (ACT), dimethylformamide (DMF) and dimethyl sulfoxide (DMSO). The maximum emission wavelengths of the dye ranged between λEM = 428 nm and 500 nm, and the photoluminescence quantum yields reached up to ϕPL = 30 % in THF solution, but also expressive values in ETAC, ACT, DMF and DMSO. The presence of water in aprotic polar solvent solutions decreased the fluorescence of 1, although significant emission intensity was still observed in THF, ACT and DMF up to 20 % water, and in DMSO with up to 20 % water. The addition of HCl to the 10 % aqueous DMSO solution induced fluorescence quenching, while under basic conditions (NaOH), the blue emission was suppressed concomitantly to a new and red-shifted emission band set at λEM = 525 nm; and this general acidochromic behavior of compound 1 in DMSO solution was also verified in THF, ACT and DMF solutions. On the other hand, the addition of HCl or NaOH to aqueous THF solution of the dye leads to fluorescence suppression in both conditions. In the biphasic systems, dye 1 was responsive to aqueous HCl when present in both TCM or EtAc organic phases, as confirmed by its fluorescence suppression in both systems, however, protonation induces a blue shifted emission band in TCM. The acidochromic behavior of dye 1 was explored by developing a simple paper-based disposable sensor for gaseous HCl and NH3. The combination of dye 1 with 3,4,5-tris(dodecyloxy)benzoic acid (TDBA) afforded the highly lipophilic supramolecular structure 1a, which surprisingly exhibited high fluorescence in DCM (ϕPL = 21 %) and DMSO (ϕPL = 38 %), as well as aqueous DMSO solutions indicates its high stability. The structural features associated with the acidochromic behavior of dye 1 as well as the formation of 1a were studied by 1H NMR. Notably, the enhanced emission of 1a in different solvent systems can be associated with the formation of stable supramolecular structures, based on hydrogen bonding-, π-stacking- and hydrophobic-combined interactions complex. Computational data supported the photochemical and acidochromic findings obtained experimentally for 1 and 1a. The results reported herein are relevant for the development of emissive organic materials and their applications, particularly in the field of chemical sensing.
咪唑菲罗啉染料及其与3,4,5-三(十二烷基氧基)苯甲酸的超分子配合物的溶剂和ph响应荧光:实验和理论见解
荧光ph响应有机结构由于其在生物和环境领域以及材料科学发展中的作用而受到广泛关注。本文研究了2-(4-(癸氧基)苯基)- 1h -咪唑[4,5-f]菲罗啉(1)的光化学和酸致变色性质。荧光光谱和裸眼分析证实,染料1呈现出溶剂依赖的发光行为,在二氯甲烷(DCM)和甲醇溶液中不发光,但在所有其他溶剂中表现出中等至强烈的荧光。在氯仿(TCM)溶液中,染料1在青色区域发出荧光,而在乙酸乙酯(EtAc)、乙腈(ACN)、四氢呋喃(THF)、丙酮(ACT)、二甲基甲酰胺(DMF)和二甲亚砜(DMSO)溶液中则观察到典型的蓝色荧光。该染料的最大发射波长范围为λEM = 428 nm ~ 500 nm,在THF溶液中的光致发光量子产率最高可达ϕPL = 30%,在ETAC、ACT、DMF和DMSO中也有表达值。在非质子极性溶剂溶液中,水的存在降低了1的荧光,尽管在含20%水的THF、ACT和DMF以及含20%水的DMSO中仍然观察到显著的发射强度。在10% DMSO水溶液中加入HCl可诱导荧光猝灭,而在碱性条件下(NaOH),蓝色发射被抑制,同时在λEM = 525 nm处形成了一个新的红移发射带;化合物1在DMSO溶液中的这种普遍的酸致变色行为在THF、ACT和DMF溶液中也得到了验证。另一方面,在染料的THF水溶液中加入HCl或NaOH会导致两种情况下的荧光抑制。在双相体系中,染料1在TCM或EtAc有机相中均对水溶液HCl有响应,证实了其在两种体系中的荧光抑制作用,然而,质子化在TCM中引起蓝移发射带。通过研制一种简单的纸基一次性气体HCl和NH3传感器,探讨了染料1的酸致变色行为。染料1与3,4,5-三(十二烷基氧基)苯甲酸(TDBA)结合形成高度亲脂的超分子结构1a,在DCM (ϕPL = 21%)和DMSO (ϕPL = 38%)中表现出高荧光,在DMSO水溶液中也表现出高稳定性。用1H NMR研究了染料1酸致变色的结构特征和1a的形成过程。值得注意的是,在不同的溶剂体系中,1a的发射增强可能与基于氢键-、π堆积-和疏水组合相互作用复合物的稳定超分子结构的形成有关。计算数据支持1和1a的光化学和酸致变色实验结果。本文所报道的结果对有机发射材料的发展及其应用,特别是在化学传感领域具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


