Mn-Mo@sulfur-dopped carbon xerogel based lamotrigine sensitive electrochemical sensor: Characterization and applications
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
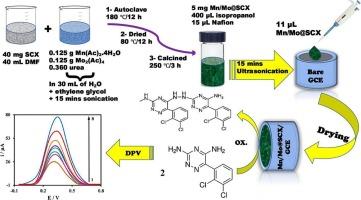
A novel low-cost voltammetric sensor based on a glassy carbon electrode (GCE) modified with sulfur-doped carbon xerogel/manganese/molybdenum (Mn-Mo@SCX/GCE) was designed for the determination of lamotrigine (LTG). The prepared nanocomposite was characterized using high-resolution transmission electron microscopy (HRTEM), scanning electron microscopy (SEM), X-ray Diffraction (XRD), Energy Dispersive X-ray spectroscopy (EDX), X-ray Photoelectron Spectroscopy (XPS), and N2-adsorption/desorption isotherm. Using electrochemical impedance spectroscopy (EIS), differential pulse voltammetry (DPV), and cyclic voltammetry (CV), the behavior of the proposed sensor for the oxidation of LTG was investigated. The obtained data revealed that Mn-Mo@SCX/GCE increased the anodic peak of LTG compared to the pristine GCE. The influence of various parameters was investigated, including nanocomposite volume, electrode surface area, scan rate, pH, and accumulation time. The optimal conditions were determined to be 12.5 μL nanocomposite, 0.13 cm2 surface area, pH 4.5, and 30 s accumulation time. Under the optimal conditions, the developed sensor exhibited a linear response toward LTG in the range of 20 nM to 43.04 μM, with a correlation coefficient of 0.9901, a limit of detection of 15 pM, and a limit of quantification of 47 pM. The analytical method validation parameters were investigated, and the developed sensor showed good accuracy, selectivity, excellent repeatability and reproducibility, and high sensitivity. Finally, the proposed analytical method was successfully applied for the quantification of LTG in its dosage forms as well as spiked human serum and urine samples, with high recovery results.
Mn-Mo@sulfur-dopped碳干凝胶基拉莫三嗪敏感电化学传感器:表征和应用
采用掺杂硫的碳干凝胶/锰/钼修饰的玻碳电极(Mn-Mo@SCX/GCE)设计了一种新型的低成本测定拉莫三嗪(LTG)的伏安传感器。采用高分辨率透射电子显微镜(HRTEM)、扫描电子显微镜(SEM)、x射线衍射(XRD)、能量色散x射线能谱(EDX)、x射线光电子能谱(XPS)和n2吸附/脱附等温线对制备的纳米复合材料进行了表征。利用电化学阻抗谱(EIS)、差分脉冲伏安法(DPV)和循环伏安法(CV)研究了所提出的传感器对LTG氧化的行为。得到的数据显示Mn-Mo@SCX/GCE与原始GCE相比,LTG的阳极峰增加。研究了纳米复合材料体积、电极表面积、扫描速率、pH和积累时间等参数对纳米复合材料的影响。最佳工艺条件为纳米复合材料12.5 μL,比表面积0.13 cm2, pH 4.5,积累时间30 s。在最优条件下,传感器对LTG在20 nM ~ 43.04 μM范围内呈线性响应,相关系数为0.9901,检测限为15 pM,定量限为47 pM。结果表明,该传感器具有良好的准确度、选择性、重复性和再现性,灵敏度高。最后,该分析方法成功地应用于LTG剂型的定量以及加标后的人血清和尿液样品,回收率高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


