Impact of Gd on the photocatalytic performance and hydrogen evaluation of the cubic phase of ZrO2
IF 3.4
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Zirconium dioxide (ZrO2) in the cubic phase has been identified as a suitable material for use as a photocatalyst. However, its wide bandgap (∼5 eV) prevents it from efficiently absorbing visible light, which is a necessity in solar-induced photocatalytic processes. In this paper, the result of gadolinium (Gd) doping on the cubic ZrO2 material in terms of structural, optical, and photocatalytic properties is examined. To make Gd-doped cubic ZrO2, the sol-gel technique was employed, and the prepared samples were characterized by XRD, scanning electron microscopy (SEM), UV–visible spectroscopy, and EIS spectroscopy. A critical evaluation of the XRD results demonstrated that the cubic phase of ZrO2 was stabilized by Gd incorporation, and the volume lattice increased from 134 to 135 Å3. Furthermore, UV–vis spectroscopy substantiated the decrease in the bandgap (from 5.0 eV for undoped ZrO2 to 4.4 eV for the 2.5 wt% Gd-doped sample), thereby facilitating enhanced visible light absorption. The photocatalytic nature of the materials was confirmed through the degradation efficiency of methyl Orange (MO) dye under a visible light source. The enhanced photocatalytic activity of the optimized Gd-doped cubic ZrO2 was observed as the degradation efficiency reached 79 % within 120 min, while that of the undoped ZrO2 was 55 %. These improved performances are due to the combined reduction of the band gap, better charge separation, and higher surface reactivity stimulated by Gd doping. This fact was reflected in the results obtained and demonstrated the prospects of using Gd-doped cubic ZrO2 as an effective photocatalyst for cleaning the environment and using solar energy. This work offers a good understanding of rare-earth doping for engineering ZrO2's properties and an effective approach for synthesizing superior photocatalysts.
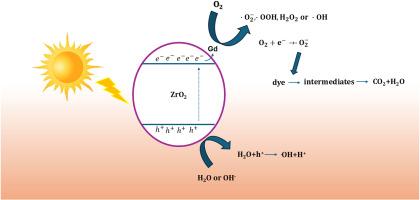
Gd对ZrO2立方相光催化性能及氢评价的影响
立方相二氧化锆(ZrO2)是一种适合用作光催化剂的材料。然而,它的宽带隙(~ 5 eV)使其无法有效吸收可见光,这在太阳能诱导的光催化过程中是必需的。本文从结构性能、光学性能和光催化性能三个方面考察了钆(Gd)在立方ZrO2材料上掺杂的结果。采用溶胶-凝胶法制备了掺杂gd的立方ZrO2,并用XRD、扫描电镜(SEM)、紫外可见光谱(UV-visible spectroscopy)和EIS光谱(EIS spectroscopy)对制备的样品进行了表征。对XRD结果的临界评价表明,Gd的加入使ZrO2的立方相稳定,体积晶格从134增加到135 Å3。此外,紫外可见光谱证实了带隙的减小(未掺杂ZrO2的带隙从5.0 eV降至2.5 wt% gd掺杂样品的4.4 eV),从而促进了可见光吸收的增强。通过在可见光下对甲基橙(MO)染料的降解效率,证实了材料的光催化性质。优化后的gd掺杂ZrO2在120 min内的光催化降解效率达到79%,而未掺杂ZrO2的光催化降解效率为55%。这些改进的性能是由于带隙的减小,更好的电荷分离,以及Gd掺杂激发的更高的表面反应性。这一事实反映在所获得的结果中,并展示了使用掺杂gd的立方ZrO2作为清洁环境和利用太阳能的有效光催化剂的前景。这项工作为工程ZrO2的稀土掺杂性能提供了很好的理解,并为合成优质光催化剂提供了有效的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


