Commensal yeast promotes Salmonella Typhimurium virulence
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
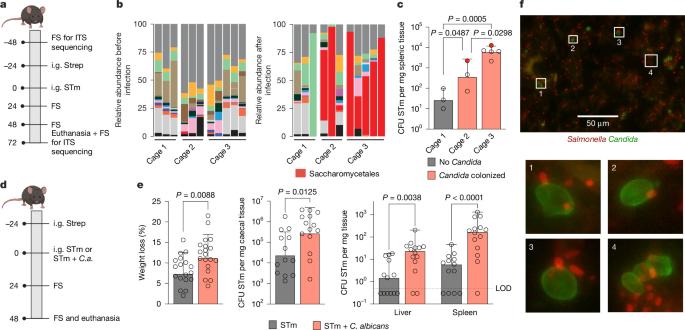
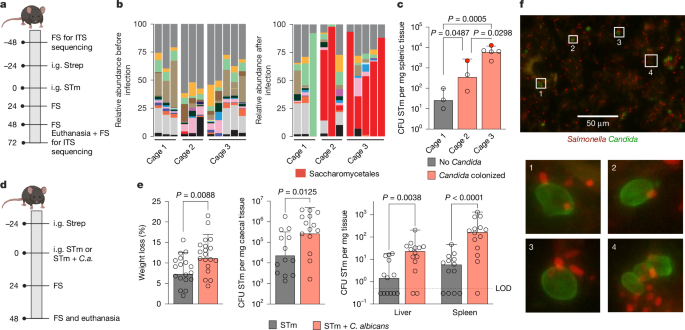
Enteric pathogens engage in complex interactions with the host and the resident microbiota to establish gut colonization1–3. Although mechanistic interactions between enteric pathogens and bacterial commensals have been extensively studied, whether and how commensal fungi affect enteric infections remain largely unknown1. Here we show that colonization with the common human gut commensal fungus Candida albicans worsened infections with the enteric pathogen Salmonella enterica subsp. enterica serovar Typhimurium. The presence of C. albicans in the mouse gut increased Salmonella caecal colonization and systemic dissemination. We investigated the underlying mechanism and found that Salmonella binds to C. albicans via type 1 fimbriae and uses its type 3 secretion system to deliver effector proteins into C. albicans. A specific effector, SopB, was sufficient to manipulate C. albicans metabolism and trigger the release of millimolar amounts of arginine into the extracellular environment. The released arginine, in turn, induced expression of the type 3 secretion system in Salmonella, increasing its invasion of epithelial cells. C. albicans deficient in arginine production was unable to increase Salmonella virulence. Arginine-producing C. albicans also dampened the inflammatory response during Salmonella infection. Arginine supplementation in the absence of C. albicans increased the systemic spread of Salmonella and decreased the inflammatory response, phenocopying the presence of C. albicans. In summary, we identified C. albicans colonization as a susceptibility factor for disseminated Salmonella infection and arginine as a central metabolite in the cross-kingdom interaction between fungi, bacteria and host. Commensal Candida albicans enhances the virulence and dissemination of Salmonella enterica subsp. enterica serovar Typhimurium.
共生酵母促进鼠伤寒沙门氏菌的毒力
肠道病原体与宿主和常驻微生物群进行复杂的相互作用,以建立肠道定植1,2,3。虽然肠道病原体和细菌共生体之间的机制相互作用已经被广泛研究,但共生真菌是否以及如何影响肠道感染在很大程度上仍然未知。在这里,我们表明,与常见的人类肠道共生真菌白色念珠菌的定植恶化了肠道病原体肠沙门氏菌亚种的感染。肠炎血清型鼠伤寒。白色念珠菌在小鼠肠道中的存在增加了沙门氏菌的盲肠定植和全身传播。我们研究了潜在的机制,发现沙门氏菌通过1型菌毛与白色念珠菌结合,并利用其3型分泌系统将效应蛋白输送到白色念珠菌体内。一个特定的效应,SopB,足以操纵白色念珠菌的代谢,并触发释放毫摩尔量的精氨酸到细胞外环境。释放的精氨酸反过来诱导沙门氏菌3型分泌系统的表达,增加其对上皮细胞的侵袭。缺乏精氨酸的白色念珠菌不能增加沙门氏菌的毒力。产生精氨酸的白色念珠菌也能抑制沙门氏菌感染期间的炎症反应。在没有白色念珠菌的情况下补充精氨酸增加了沙门氏菌的全身传播,降低了炎症反应,表型复制了白色念珠菌的存在。总之,我们确定白色念珠菌定植是播散性沙门氏菌感染的易感因素,精氨酸是真菌、细菌和宿主跨界相互作用的中心代谢物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


