Tau accelerates tubulin exchange in the microtubule lattice
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
Microtubules are cytoskeletal filaments characterized by dynamic instability at their tips and a dynamic lattice that undergoes continuous tubulin loss and incorporation. Tau, a neuronal microtubule-associated protein, is well known for its role in stabilizing microtubule tips and promoting microtubule bundling. Here we demonstrate that tau also modulates microtubule lattice dynamics. Although tau lacks enzymatic activity, it significantly accelerates tubulin exchange within the lattice, particularly at topological defect sites. Our findings indicate that tau enhances lattice anisotropy by stabilizing longitudinal tubulin–tubulin interactions while destabilizing lateral ones, thereby enhancing the mobility and annihilation of lattice defects. These results challenge the traditional view of tau as merely a passive stabilizer, revealing its active role in dynamically remodelling the microtubule lattice structure. Beyond its known role in stabilizing microtubules, it is now shown that tau protein actively promotes lattice defect repair by enhancing tubulin turnover at topological defects.
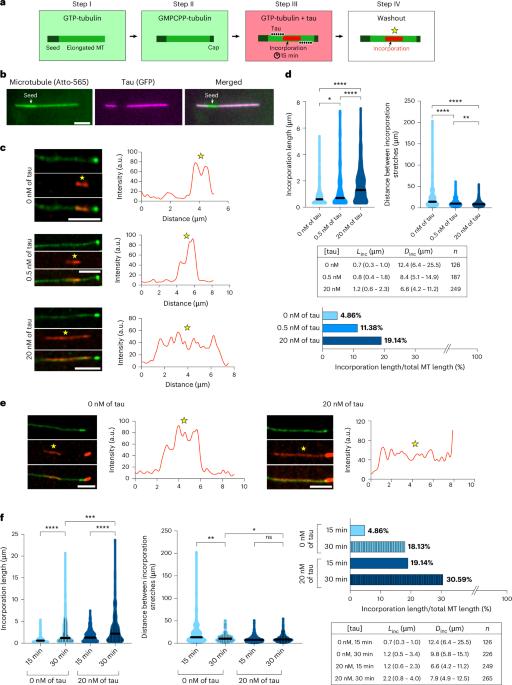

Tau蛋白加速微管晶格中的微管蛋白交换
微管是细胞骨架细丝,其特征是其尖端的动态不稳定和动态晶格,经历连续的微管蛋白损失和合并。Tau是一种神经元微管相关蛋白,以其稳定微管尖端和促进微管捆绑的作用而闻名。在这里,我们证明了tau也调节微管晶格动力学。虽然tau缺乏酶活性,但它显著加速了晶格内的微管蛋白交换,特别是在拓扑缺陷位点。我们的研究结果表明,tau通过稳定纵向微管蛋白与微管蛋白的相互作用来增强晶格的各向异性,同时破坏横向的相互作用,从而增强晶格缺陷的迁移性和湮灭性。这些结果挑战了tau蛋白仅仅作为被动稳定剂的传统观点,揭示了其在动态重塑微管晶格结构中的积极作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


