An anisotropic cardiac patch with barbed microneedles for enhanced tissue anchorage and myocardial repair
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Microneedle patches can penetrate the myocardium to facilitate integration with cardiac tissue, offering a promising approach for myocardial infarction (MI) repair. However, their clinical translation has been hindered by insufficient fixation stability during cardiac contractions and mismatch with myocardial anisotropy. To address these challenges, a bioinspired three-dimensional cardiac patch integrating barbed microneedles and an anisotropic lightweight mesh was designed. The microneedle with outward-expanding barbs (OEBMN) demonstrated 10.8-fold stronger tissue anchorage than the barbless microneedle, while achieving a 6-fold reduction in the force ratio (insertion force/pulling-out force), indicating an enhanced anchoring performance. The OEBMN enabled sutureless patch transplantation onto the epicardium, ensuring more uniform stress distribution than suture-fixation systems where stress concentrations typically occur at knotting sites. The knitted mesh exhibited sufficient strength to provide long-term mechanical compensation to the infarcted myocardium, whereas the honeycomb-like structure satisfied the native myocardial anisotropy. Furthermore, the cardiac patch promoted comprehensive mechanical integration with the infarcted heart through OEBMNs, enabling multi-directional support from the inner myocardium to the epicardium. The rat MI model experiments revealed that the patch not only improved cardiac function and electrophysiological characteristics but also increased ventricular wall thickness, reduced fibrosis, and promoted angiogenesis. Transcriptome sequencing revealed that the potential mechanisms by which the patch promotes myocardial repair mainly include inhibiting apoptosis- and fibrosis-related pathways. Overall, this study proposes a sutureless fixation strategy for cardiac patches and highlights the latent potential of providing anisotropic mechanical support for MI repair.
Statement of significance
Microneedle patches offer a promising platform for myocardial repair by directly penetrating cardiac tissue and enabling mechanical integration with the tissue. However, their application is hindered by limited fixation stability and structural mismatch with anisotropic myocardium. Herein, a cardiac patch combining an anisotropic lightweight mesh with microneedles featuring outward-expanding barbs was constructed. This design allows stable anchoring, sutureless implantation with reduced insertion force, uniform stress transmission, and anisotropic mechanical support to inhibit ventricular dilation. In vivo results showed that the patch significantly enhanced cardiac contractile function, reduced ventricular fibrosis, and increased ventricular wall thickness. This unique design demonstrates substantial potential for sutureless fixation scenarios and the repair of infarcted myocardium.
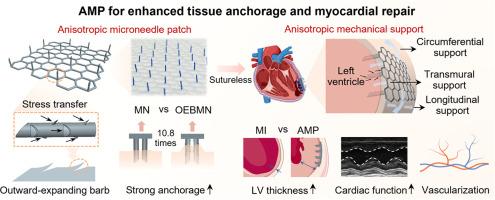
带倒刺微针的各向异性心脏贴片,用于增强组织锚定和心肌修复。
微针贴片可以穿透心肌,促进与心脏组织的整合,为心肌梗死(MI)修复提供了一种有前景的方法。然而,由于心脏收缩时固定稳定性不足以及与心肌各向异性不匹配,它们的临床转化受到阻碍。为了解决这些挑战,设计了一种生物启发的三维心脏贴片,该贴片集成了倒刺微针和各向异性轻质网格。外展倒刺微针(OEBMN)的组织锚定强度是无倒刺微针的10.8倍,而力比(插入力/拔出力)降低了6倍,表明锚定性能增强。OEBMN使无缝线贴片移植到心外膜上,确保比缝线固定系统更均匀的应力分布,缝线固定系统的应力集中通常发生在打结部位。编织网具有足够的强度为梗死心肌提供长期的机械补偿,而蜂窝状结构满足心肌的天然各向异性。此外,心脏贴片通过oebmn促进了与梗死心脏的全面机械整合,实现了从心肌内膜到心外膜的多向支持。大鼠心肌梗死模型实验表明,该贴片不仅改善了心功能和电生理特性,而且增加了心室壁厚度,减少了纤维化,促进了血管生成。转录组测序显示,该贴片促进心肌修复的潜在机制主要包括抑制凋亡和纤维化相关途径。总之,本研究提出了心脏补片的无缝线固定策略,并强调了为心肌梗死修复提供各向异性机械支持的潜在潜力。意义声明:微针贴片通过直接穿透心脏组织并实现与组织的机械整合,为心肌修复提供了一个有前景的平台。然而,它们的应用受到固定稳定性有限和与各向异性心肌结构不匹配的阻碍。本文构建了一种由各向异性轻质网状结构和向外扩展倒刺的微针组成的心脏贴片。这种设计允许稳定的锚定,减少插入力的无缝线植入,均匀的应力传递和各向异性的机械支持来抑制心室扩张。体内实验结果显示,该贴片可显著增强心脏收缩功能,减少心室纤维化,增加心室壁厚度。这种独特的设计为无缝线固定和梗死心肌修复提供了巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


