Human induced pluripotent stem cell-derived nanovesicles for the treatment of ischemic limb disease
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
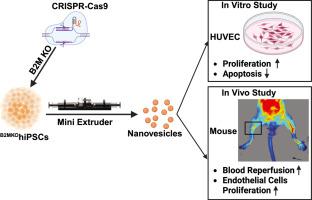
Critical limb ischemia is an advanced stage of peripheral artery disease, characterized by claudication, ischemic pain, and ulceration. It is a severe condition associated with an increased risk of limb amputation and mortality. Although extracellular vehicles (EVs) secreted by endothelial cells (ECs) or mesenchymal stem cells (MSCs) have shown promise for the treatment of ischemic limb diseases in mice, clinical translation has been limited by the low EV yields from cultured cells. In this study, a hypo-immunogenic human induced pluripotent stem cell (hiPSC) line with β2-microglobulin knockout (B2MKOhiPSC) was used to manufacture nanovesicles (B2MKOhiPSC![]() NVs), which are hypo-immunogenic as compared to wild type hiPSC manufactured nanovesicles (WThiPSC
NVs), which are hypo-immunogenic as compared to wild type hiPSC manufactured nanovesicles (WThiPSC![]() NVs). Notably, over 9500 NVs could be produced from a single hiPSC. The zeta potential of hiPSC
NVs). Notably, over 9500 NVs could be produced from a single hiPSC. The zeta potential of hiPSC![]() NVs was -16.7 mV, as measured by the ZETASIZER Nano series instrument. The NVs exhibited a mean diameter of 115.9 ± 43.5 nm, as determined by Nanosight analysis, and displayed bilayer lipid membranes under transmission electron microscopy. In vitro, both WThiPSC
NVs was -16.7 mV, as measured by the ZETASIZER Nano series instrument. The NVs exhibited a mean diameter of 115.9 ± 43.5 nm, as determined by Nanosight analysis, and displayed bilayer lipid membranes under transmission electron microscopy. In vitro, both WThiPSC![]() NVs and B2MKOhiPSC
NVs and B2MKOhiPSC![]() NVs protected human umbilical vein endothelial cells (HUVECs) from hypoxic injury, promoted HUVEC proliferation, and did not induce hemolysis. In vivo, B2MKOhiPSC
NVs protected human umbilical vein endothelial cells (HUVECs) from hypoxic injury, promoted HUVEC proliferation, and did not induce hemolysis. In vivo, B2MKOhiPSC![]() NVs administration significantly improved blood perfusion in ischemic limbs 14 days post-treatment. This was accompanied by a notable increase in mouse endothelial cell (EC) proliferation and neovascularization compared to C57BL mice treated with either a saline injection or WThiPSC
NVs administration significantly improved blood perfusion in ischemic limbs 14 days post-treatment. This was accompanied by a notable increase in mouse endothelial cell (EC) proliferation and neovascularization compared to C57BL mice treated with either a saline injection or WThiPSC![]() NVs, without the use of immunosuppressive drugs. Furthermore, intramuscular injections of WThiPSC
NVs, without the use of immunosuppressive drugs. Furthermore, intramuscular injections of WThiPSC![]() NVs did not cause adverse effects on liver or kidney function. These findings suggest that B2MKOhiPSC- NVs represent a promising allogeneic therapeutic approach for the treatment of ischemic limb diseases.
NVs did not cause adverse effects on liver or kidney function. These findings suggest that B2MKOhiPSC- NVs represent a promising allogeneic therapeutic approach for the treatment of ischemic limb diseases.
Statement of significance
The use of hiPSCs as parental cells can easily scale-up NV production.
NVs derived from β2 microglobulin-knockout hiPSCs (B2MKOhiPSC![]() NVs) protect endothelial cells from hypoxic injury and enhance their proliferation.
NVs) protect endothelial cells from hypoxic injury and enhance their proliferation.
In immunocompetent mice, administration of B2MKOhiPSC![]() NVs significantly enhanced blood perfusion and promoted neovascularization in ischemic limbs.
NVs significantly enhanced blood perfusion and promoted neovascularization in ischemic limbs.
人诱导多能干细胞衍生的纳米囊泡治疗缺血性肢体疾病。
危急肢体缺血是外周动脉疾病的晚期,以跛行、缺血性疼痛和溃疡为特征。这是一种严重的疾病,与截肢和死亡风险增加有关。虽然内皮细胞(ECs)或间充质干细胞(MSCs)分泌的细胞外载体(EVs)已显示出治疗小鼠缺血性肢体疾病的希望,但由于培养细胞的EVs产量低,临床转化受到限制。在这项研究中,用β2-微球蛋白敲除(B2MKOhiPSC)的低免疫原性人诱导多能干细胞(hiPSC)系来制造纳米囊泡(B2MKOhiPSC- nvs),与野生型hiPSC制造的纳米囊泡(WThiPSC-NVs)相比,其免疫原性较低。值得注意的是,单个hiPSC可以产生超过9,500个NVs。通过ZETASIZER纳米系列仪器测量,hiPSC-NVs的zeta电位为-16.7 mV。通过纳米视觉分析,NVs的平均直径为115.9±43.5 nm,透射电镜下显示双层脂质膜。在体外,WThiPSC-NVs和B2MKOhiPSC-NVs均能保护人脐静脉内皮细胞(HUVEC)免受缺氧损伤,促进HUVEC增殖,且不诱导溶血。在体内,给药后14天,B2MKOhiPSC-NVs显著改善缺血肢体的血液灌注。与不使用免疫抑制药物的生理盐水注射或WThiPSC-NVs治疗的C57BL小鼠相比,小鼠内皮细胞(EC)增殖和新生血管明显增加。此外,肌肉注射WThiPSC-NVs对肝肾功能没有不良影响。这些发现表明,B2MKOhiPSC- NVs是治疗缺血性肢体疾病的一种有前途的同种异体治疗方法。意义声明:使用hiPSCs作为亲代细胞可以很容易地扩大NV的生产。来源于β2微球蛋白敲除hipsc (B2MKOhiPSC-NVs)的NVs可以保护内皮细胞免受缺氧损伤并促进其增殖。在免疫正常小鼠中,给予B2MKOhiPSC-NVs可显著增强缺血肢体的血液灌注,促进新生血管的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


