Region-specific gene expression and sex inform about disease susceptibility in the aorta
IF 10.8
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
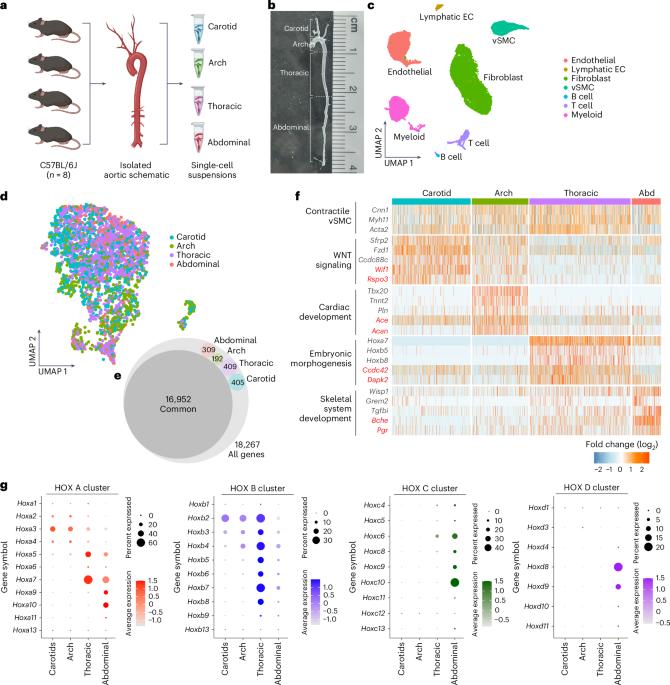
Pathology in large vessels frequently develops at specific locations, implying that local stressors and spatially restricted gene expression are likely contributors to disease susceptibility. Here we perform single-cell transcriptomics in the carotids, the aortic arch and the thoracic and abdominal aorta to identify site- and sex-specific differences that could inform about vulnerability. Our findings revealed (1) regionally defined transcriptional profiles, (2) signatures associated with embryonic origins and (3) differential contributions of sex-specific effectors. Furthermore, cross-referencing regional-specific signatures with available genome-wide association study and expression quantitative trait loci databases identified 339 disease candidates associated with aorta distensibility, stiffness index and blood pressure. CPNE8 and SORBS2 were further evaluated and highlighted as strong causal candidates. Sex differences were predominantly observed in the thoracic and abdominal aorta. MCAM (CD146), a transcript with sex-skewed expression and lower in male mice and men, had significantly reduced expression in human aortic aneurysms. The findings reveal underlying diversity within vascular smooth muscle cell populations relevant to understanding site-specific and sex-specific variation of vascular pathologies. Single-cell profiling reveals regional and sex-specific transcriptional programs in the aorta, uncovering molecular diversity that may drive site-selective and sex-biased vulnerability to aneurysms.
区域特异性基因表达和性别提示主动脉疾病易感性。
大血管病变经常发生在特定部位,这意味着局部应激源和空间限制性基因表达可能是疾病易感性的因素。在这里,我们对颈动脉、主动脉弓、胸主动脉和腹主动脉进行单细胞转录组学研究,以确定部位和性别特异性差异,从而了解易感性。我们的发现揭示了(1)区域定义的转录谱;(2)与胚胎起源相关的特征;(3)性别特异性效应物的差异贡献。此外,交叉参考区域特异性特征与可用的全基因组关联研究和表达定量性状位点数据库,确定了339种与主动脉扩张度、僵硬指数和血压相关的疾病候选者。CPNE8和SORBS2被进一步评估并强调为强有力的因果候选。性别差异主要见于胸主动脉和腹主动脉。MCAM (CD146)是一种性别倾斜表达的转录物,在雄性小鼠和男性中表达较低,在人类主动脉瘤中表达显著降低。研究结果揭示了血管平滑肌细胞群的潜在多样性,这与理解血管病理的位点特异性和性别特异性变异有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


