Nanocrystal synthesis with alkoxy reagents for dispersion in polar and non-polar solvents
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
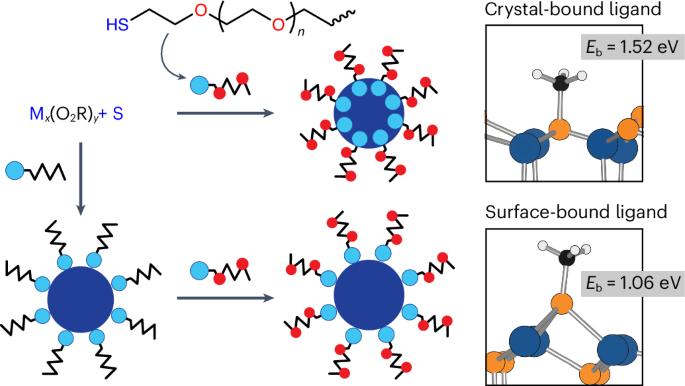
Applications of colloidal nanocrystals in polar solvents often require nanocrystals synthesized in non-polar solvents. However, solvent transfer processes are problematic and deteriorate nanocrystal quality. Here we report syntheses of nanocrystals with nearly universal solvent dispersibility using ligands and solvents with alkoxy repeating units. Core syntheses, shell deposition and cation exchange proceed similarly to traditional methods while products are more stable in aqueous solution than those generated by solvent transfer. (CdSe)CdZnS nanocrystals retain photoluminescence in cells for single-particle tracking experiments and outperform other nanocrystal classes in diffusion metrics reflecting stability and resistance to non-specific binding. Distinct reaction classes yield nanocrystals with either methoxy or hydroxy ligand terminations, both of which can be purified by aqueous methods that are chemically greener than traditional methods. These reactions can further generate nanocrystals with diverse oxide, sulfide and selenide compositions, shapes and spectral bands with wide dispersibility that may make applications in polar solvents more widely accessible. Nanocrystals are synthesized with long-term colloidal stability in both polar and non-polar solvents without the need for ligand exchange. During synthesis, conventional coordinating ligands and solvents with long alkyl chains are replaced with those bearing alkoxy repeating units.
用烷氧基试剂合成纳米晶体,用于极性和非极性溶剂的分散。
胶体纳米晶体在极性溶剂中的应用往往需要在非极性溶剂中合成纳米晶体。然而,溶剂转移工艺是有问题的,并且会降低纳米晶体的质量。在这里,我们报道了使用具有烷氧基重复单元的配体和溶剂合成具有几乎普遍溶剂分散性的纳米晶体。核合成、壳沉积和阳离子交换的过程与传统方法相似,产物在水溶液中比溶剂转移生成的产物更稳定。(CdSe)CdZnS纳米晶体在细胞中保持光致发光,用于单粒子跟踪实验,并且在反映稳定性和抵抗非特异性结合的扩散指标方面优于其他纳米晶体类别。不同的反应类别产生具有甲氧基或羟基配体末端的纳米晶体,这两种纳米晶体都可以用比传统方法更环保的水方法纯化。这些反应可以进一步产生具有多种氧化物、硫化物和硒化物组成、形状和光谱带的纳米晶体,具有广泛的分散性,这可能使极性溶剂的应用更加广泛。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


