Human ocular fluid outflow on-chip reveals trabecular meshwork-mediated Schlemm’s canal endothelial dysfunction in steroid-induced glaucoma
IF 10.8
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Glaucoma is a leading cause of blindness, featuring elevated intraocular pressure and consequential optic nerve damage1. While elevated intraocular pressure is due to impaired ocular fluid outflow through both the trabecular meshwork (TM) and the lymphatic-like Schlemm’s canal (SC) endothelium, the mechanism by which SC endothelium regulates fluid outflow in cooperation with the TM in healthy and glaucomatous conditions remains unclear. Here we create a human ocular fluid outflow on-chip, composed of a three-dimensional lymphatic or SC endothelium surrounded by TM and draining interstitial fluid. Using the system, we recapitulate steroid-induced glaucoma, characterized by decreased fluid outflow and tightened SC endothelial junctions. We further reveal that the glaucoma phenotypes are induced by an ALK5/VEGFC-mediated SC endothelial dysfunction in the presence of TM. The ocular fluid outflow on-chip provides a unique platform for bridging traditional in vitro and in vivo models of ocular lymphatic physiology and disease. Lu et al. created a human ocular outflow on-chip, composed of 3D Schlemm’s canal endothelium surrounded by trabecular meshwork and draining interstitial fluid, revealing ALK5/VEGFC signaling as a therapeutic target for steroid-induced glaucoma.
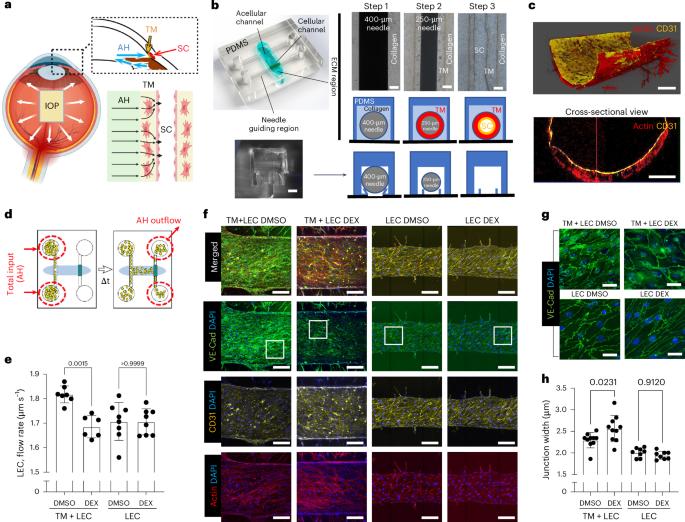
芯片显示激素性青光眼中小梁网介导的施莱姆管内皮功能障碍。
青光眼是致盲的主要原因,以眼压升高和视神经损伤为特征。虽然眼压升高是由于眼液通过小梁网(TM)和淋巴样施莱姆管(SC)内皮流出受损所致,但在健康和青光眼情况下,SC内皮与小梁网协同调节液体流出的机制尚不清楚。在这里,我们在芯片上创建了一个人眼液体流出,由TM包围的三维淋巴或SC内皮组成,并排出间质液。使用该系统,我们概括了类固醇诱导的青光眼,其特征是液体流出减少和SC内皮连接收紧。我们进一步发现,在TM存在的情况下,ALK5/ vegf介导的SC内皮功能障碍可诱导青光眼表型。芯片上的眼液流出为桥接传统的体外和体内眼淋巴生理和疾病模型提供了一个独特的平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


