Identification of a novel insecticidal chlorotoxin-like peptide from the venom of the Compsobuthus egyptiensis scorpion
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Scorpion venom contains various bioactive peptides, but there are many scorpion species whose venom has not been studied. The genus Compsobuthus, belonging to the family Buthidae, is relatively diverse, but there have been no reports on their venom components. In the present study, we characterized venom components of the Compsobuthus egyptiensis scorpion inhabiting the northern Egyptian desert. Mass spectrometry analysis of the venom revealed that the components with molecular masses from 3000 to 4000 Da were relatively abundant among 198 components detected. We then isolated a novel insecticidal peptide, Ce-1, from one of the HPLC fractions showing insecticidal activity. The structure of Ce-1 was determined using a combination of Edman degradation and de novo MS/MS sequencing analyses. This revealed that Ce-1 consists of 36 amino acid residues with four disulfide bonds. The deduced structure was confirmed by comparison with the synthetic peptide. Ce-1 shares high sequence homology to chlorotoxin-like peptides, which consist of an α-helix and an antiparallel triple-stranded β-sheet cross-linked by four disulfide bonds. Future research on Ce-1 will contribute to elucidating the mechanism of action of insecticidal chlorotoxin-like peptides.
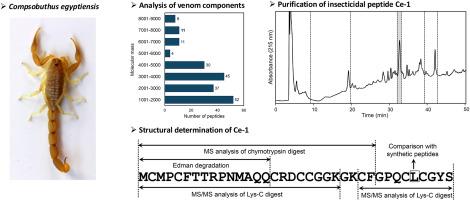
埃及蝎毒液中一种新型杀虫氯毒素样肽的鉴定。
蝎子毒液含有多种生物活性肽,但有许多种类的蝎子毒液尚未被研究过。隶属于Buthidae科的Compsobuthus属种类繁多,但尚未见有关其毒液成分的报道。在本研究中,我们对居住在埃及北部沙漠的埃及蝎的毒液成分进行了表征。质谱分析表明,在检测到的198种成分中,分子质量在3000 ~ 4000 Da的成分相对较多。然后,我们从一个显示杀虫活性的HPLC组分中分离出一种新的杀虫肽Ce-1。Ce-1的结构采用Edman降解和de novo MS/MS测序分析相结合的方法确定。这表明Ce-1由36个氨基酸残基和4个二硫键组成。通过与合成肽的比较,证实了推导出的结构。Ce-1与氯毒素样肽具有高度的序列同源性,氯毒素样肽由α-螺旋和反平行的三链β-片组成,由四个二硫键交联。对Ce-1的进一步研究将有助于阐明氯毒素样杀虫肽的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


