Towards model-informed precision dosing of cyclosporine in adult renal transplantation: Assessing population pharmacokinetic models and multi-model strategies
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background
Cyclosporine’s narrow therapeutic index and pharmacokinetic variability challenge its optimal dosing in renal transplantation. Model-informed precision dosing (MIPD), utilizing population pharmacokinetic (popPK) models and Bayesian forecasting, can enhance dosing optimization and improve clinical outcomes. This study evaluated the predictive performance of published popPK models and multi-model strategies for cyclosporine, using therapeutic drug monitoring (TDM) data and full pharmacokinetic (PK) profiles.
Methods
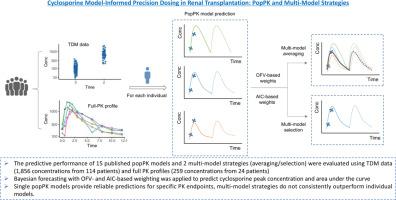
We evaluated 15 published popPK models and 2 multi-model strategies (averaging/selection) using TDM data (1,856 concentrations from 114 patients) and full-PK profiles (259 concentrations from 24 patients). Bayesian forecasting with objective function value (OFV)- and Akaike information criterion (AIC)-based weighting was applied to predict cyclosporine peak concentration (C₂) and area under the curve (AUC). Predictive accuracy and precision were assessed using relative bias (rBias) and relative root mean square error (rRMSE).
Results
The two-compartment model with first-order absorption and transit compartments (Press et al., 2010) provided the best prediction for C₂ (rBias = -1.40%, rRMSE = 5.38%), while the model with lag time and covariates postoperative days, age, and weight (Baek et al., 2014) excelled in AUC prediction (rBias = 4.82%, rRMSE = 1.92%). Multi-model averaging/selection performed comparably to top-performing single popPK models for C₂ (rBias <10% with OFV-based weighting) but underperformed for AUC prediction (rBias >20%).
Conclusion
While single popPK models provide reliable predictions for specific PK endpoints, multi-model strategies do not consistently outperform individual models. The optimal model selection for MIPD should be fit-for-purpose to ensure optimal cyclosporine dosing and improved clinical outcomes in renal transplantation.
基于模型的环孢素成人肾移植精确给药:评估群体药代动力学模型和多模型策略。
背景:环孢素狭窄的治疗指数和药代动力学变异性对其在肾移植中的最佳剂量提出了挑战。基于群体药代动力学(popPK)模型和贝叶斯预测的模型知情精确给药(MIPD)可以优化给药效果,改善临床疗效。本研究利用治疗药物监测(TDM)数据和完整药代动力学(PK)谱,评估了已发表的popPK模型和多模型策略对环孢素的预测性能。方法:我们使用TDM数据(114例患者的1856个浓度)和全pk谱(24例患者的259个浓度)评估了15个已发表的popPK模型和2个多模型策略(平均/选择)。采用基于目标函数值(OFV)和赤池信息准则(AIC)加权的贝叶斯预测方法预测环孢素峰值浓度(C₂)和曲线下面积(AUC)。采用相对偏倚(rBias)和相对均方根误差(rRMSE)评估预测的准确度和精密度。结果:含有一阶吸收室和运输室的两室模型(Press et al., 2010)对C₂的预测效果最好(rBias = -1.40%,rRMSE = 5.38%),而含有滞后时间和术后时间、年龄和体重协变量的模型(Baek et al., 2014)对AUC的预测效果较好(rBias = 4.82%,rRMSE = 1.92%)。对于C₂(rBias为20%),多模型平均/选择的表现与表现最好的单popPK模型相当。结论:虽然单一popPK模型为特定的PK端点提供了可靠的预测,但多模型策略并不总是优于单个模型。MIPD的最佳模型选择应符合目的,以确保最佳环孢素剂量和改善肾移植的临床结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


