Multilineage potential induced by reconstruction of three-dimensional structures in mouse fetal brain cells
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
We examined whether the multilineage potential of mouse fetal brain cells is induced by the three-dimensional (3D) structural reconstruction. Dissociated single cells from the cerebral hemispheres of mouse fetuses at embryonic day 14.5–15 (E14.5–15) rapidly aggregated into cell clusters, forming spherical cell aggregates (SCAs). Immunofluorescence staining revealed that SCAs were positive for Map2 but negative for Nestin, GFAP, and markers of undifferentiated cells such as Oct4, SSEA-1, ALDH2, and CD133. During culture of SCAs in embryonic stem cell medium, SCAs subsequently developed into embryoid bodies (EBs), which began to exhibit positive immunofluorescence staining for Oct4. Reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting analyses further confirmed the expression of Oct4 and Nanog at the gene and protein levels. Differentiated cells of EBs induced by culture in various types of media showed positive immunofluorescence staining for lineage-specific markers, including AFP, α-SMA, GFAP, Map2 and C-peptide and expressed the corresponding genes and proteins. These results suggest that mouse fetal brain cells can regain multilineage potential through the reconstruction of 3D structures without genetic modification. Our findings indicate that 3D culture systems may support non-genetic reprogramming strategies in regenerative medicine by activating intrinsic plasticity of cells.
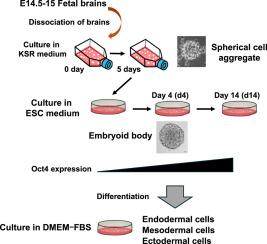
重建小鼠胎儿脑细胞三维结构诱导的多谱系电位。
我们研究了三维(3D)结构重建是否会诱导小鼠胎儿脑细胞的多谱系电位。胚胎期14.5-15天(E14.5-15)小鼠大脑半球分离的单细胞迅速重新聚集成细胞簇,形成球形细胞聚集体(SCAs)。免疫荧光染色显示,SCAs中Map2呈阳性,而Nestin、GFAP和未分化细胞标志物Oct4、SSEA-1、ALDH2和CD133呈阴性。在胚胎干细胞培养基中培养SCAs时,SCAs随后发育成胚状体(EBs),胚状体开始表现出Oct4免疫荧光染色阳性。逆转录聚合酶链反应(RT-PCR)和Western blotting进一步证实了Oct4和Nanog在基因和蛋白水平上的表达。在不同培养基培养诱导的EBs分化细胞中,AFP、α-SMA、GFAP、Map2、c肽等谱系特异性标志物的免疫荧光染色均呈阳性,并表达相应的基因和蛋白。这些结果表明,小鼠胎儿脑细胞可以通过重建3D结构而无需基因修饰来重新获得多谱系潜力。我们的研究结果表明,3D培养系统可以通过激活细胞的内在可塑性来支持再生医学中的非遗传重编程策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


