Systemic lupus erythematosus and the gut microbiome: To look forward is to look within – A systematic review and narrative synthesis
IF 8.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Background
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease shaped by complex interactions involving genetic and environmental factors. Among these, the gut microbiome is emerging as potentially modulating immune responses and influencing disease susceptibility, progression, and activity.
Objectives
To synthesize current evidence on gut microbiome changes in adult SLE patients, framed along the clinical pathway – from diagnosis to treatment – to help bridge bench and bedside for microbiome-informed SLE care and research.
Methods
A systematic search identified primary research studies examining gut microbiota in adult SLE patients. Studies were reviewed in a stepwise manner by independent investigators. Findings were synthesized narratively, emphasizing human data.
Results
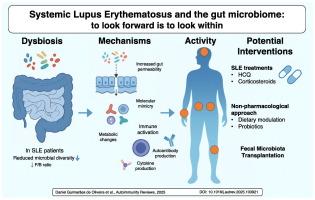
SLE patients exhibit gut microbiome dysbiosis, with reduced microbial richness and altered bacterial taxa. A lower Firmicutes/Bacteroidetes ratio is frequently observed. Enrichment of specific taxa, such as Enterococcus, Lactobacillus, and Ruminococcus gnavus, is reported. Dysbiosis correlates with increased gut permeability, immune activation, and autoreactivity. Clinical associations include disease activity, flares, nephritis, and other manifestations. SLE treatments, such as hydroxychloroquine and corticosteroids, influence the microbiome. Emerging interventions such as dietary modulation and fecal microbiota transplantation show promise in early studies. However, considerable heterogeneity exists across studies in terms of patient characteristics, methodology, and taxa-level findings.
Conclusions
The gut microbiome has multifaceted associations with SLE pathogenesis, disease activity, and therapeutic response. Translation will require standardized methods, functional validation, longitudinal follow-up, and clinical integration. While uncertainties remain, the gut microbiome is increasingly relevant, and clinicians caring for patients with SLE should be aware of its emerging implications.
系统性红斑狼疮和肠道微生物组:展望未来就是展望未来——系统回顾和叙述综合。
背景:系统性红斑狼疮(SLE)是一种异质性自身免疫性疾病,涉及遗传和环境因素的复杂相互作用。其中,肠道微生物群正在成为调节免疫反应和影响疾病易感性、进展和活动的潜在因素。目的:综合目前关于成年SLE患者肠道微生物组变化的证据,沿着临床途径(从诊断到治疗)构建框架,以帮助架起实验室和床边的桥梁,为微生物组知情的SLE护理和研究。方法:系统搜索确定了检查成年SLE患者肠道微生物群的初步研究。研究由独立调查人员逐步进行审查。研究结果综合叙述,强调人的数据。结果:SLE患者表现出肠道菌群失调,微生物丰富度降低,细菌类群改变。经常观察到较低的厚壁菌门/拟杆菌门比率。据报道,特定分类群如肠球菌、乳酸杆菌和瘤球菌的富集。生态失调与肠道通透性、免疫激活和自身反应性增加有关。临床关联包括疾病活动性、耀斑、肾炎和其他表现。SLE治疗,如羟氯喹和皮质类固醇,会影响微生物组。新兴的干预措施,如饮食调节和粪便微生物群移植在早期研究中显示出希望。然而,在患者特征、方法学和分类水平的发现方面,各研究存在相当大的异质性。结论:肠道微生物组与SLE发病机制、疾病活动性和治疗反应具有多方面的关联。翻译将需要标准化的方法、功能验证、纵向随访和临床整合。虽然不确定性仍然存在,但肠道微生物组的相关性越来越大,治疗SLE患者的临床医生应该意识到其新出现的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Autoimmunity reviews
医学-免疫学
CiteScore
24.70
自引率
4.40%
发文量
164
审稿时长
21 days
期刊介绍:
Autoimmunity Reviews is a publication that features up-to-date, structured reviews on various topics in the field of autoimmunity. These reviews are written by renowned experts and include demonstrative illustrations and tables. Each article will have a clear "take-home" message for readers.
The selection of articles is primarily done by the Editors-in-Chief, based on recommendations from the international Editorial Board. The topics covered in the articles span all areas of autoimmunology, aiming to bridge the gap between basic and clinical sciences.
In terms of content, the contributions in basic sciences delve into the pathophysiology and mechanisms of autoimmune disorders, as well as genomics and proteomics. On the other hand, clinical contributions focus on diseases related to autoimmunity, novel therapies, and clinical associations.
Autoimmunity Reviews is internationally recognized, and its articles are indexed and abstracted in prestigious databases such as PubMed/Medline, Science Citation Index Expanded, Biosciences Information Services, and Chemical Abstracts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


