Construction of functionalized adjacent P(v)–C chiral stereogenic centers via organophosphine-catalyzed asymmetric SN2′ substitution of unsymmetrical phosphine oxides with MBH adducts
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Phosphorus(v) stereogenic centers are prevalent in various pharmaceuticals, pesticides, and functional materials. Herein, we report an organophosphine-catalyzed asymmetric SN2′ substitution between unsymmetrical phosphine oxides and Morita–Baylis–Hillman adducts through an umpolung addition pathway, allowing the efficient construction of functionalized adjacent P(v)–C chiral stereogenic centers. A chiral phosphine catalyst, derived from an amino acid, enabled the formation of the desired products in high yields (up to 99%) and excellent enantioselectivities (up to 97% ee). This method demonstrates broad substrate scope, and the products can be easily diversified into valuable derivatives.
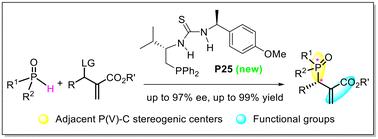
用MBH加合物催化不对称SN2取代不对称膦氧化物构建功能化邻P(V)-C手性立体中心
磷(V)立体中心普遍存在于各种药物、农药和功能材料中。在此,我们报道了一种有机膦催化的不对称SN2'取代,通过非对称加成途径在不对称膦氧化物和morta - bayis - hillman加合物之间进行取代,从而有效地构建了官能化相邻的P(V) c手性立体中心。一种源自氨基酸的手性膦催化剂,能够以高收率(高达99%)和优异的对映选择性(高达97% ee)形成所需的产物。该方法具有广泛的底物范围,产品可以很容易地多样化为有价值的衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


