Cross-feeding percolation phase transitions of intercellular metabolic networks
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Intercellular cross-talk is essential for the adaptation capabilities of populations of cells. While direct diffusion-driven cell-to-cell exchanges are difficult to map, current nanotechnology enables one to probe single-cell exchanges with the medium. We introduce a mathematical method to reconstruct the dynamic unfolding of intercellular exchange networks from these data, applying it to an experimental coculture system. The exchange network, initially dense, progressively fragments into small disconnected clusters. To explain these dynamics, we develop a maximum-entropy multicellular metabolic model with diffusion-driven exchanges. The model predicts a transition from a dense network to a sparse one as nutrient consumption shifts. We characterize this crossover both numerically, revealing a power-law decay in the cluster-size distribution, and analytically, by connecting to percolation theory. Comparison with data suggests that populations evolve toward the sparse phase by remaining near the crossover. These findings offer insights into the collective organization driving the adaptive dynamics of cell populations.
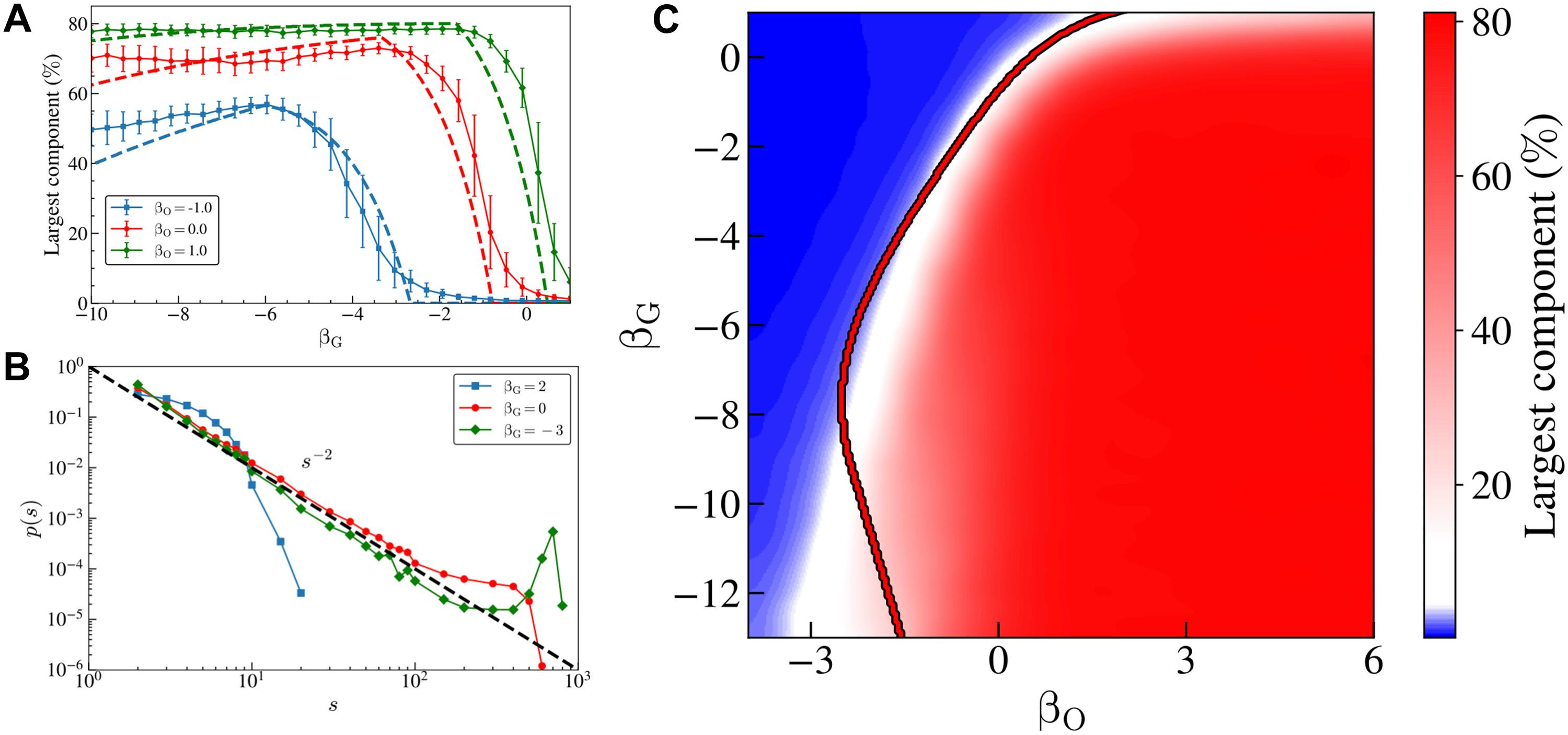
细胞间代谢网络的交叉摄食渗透相变
细胞间串扰对细胞群体的适应能力至关重要。虽然直接扩散驱动的细胞间交换很难绘制,但目前的纳米技术使人们能够探测单细胞与介质之间的交换。我们引入了一种数学方法,从这些数据中重建细胞间交换网络的动态展开,并将其应用于实验共培养系统。交换网络,最初密集,逐渐分裂成小的不相连的集群。为了解释这些动态,我们开发了一个具有扩散驱动交换的最大熵多细胞代谢模型。该模型预测,随着营养消耗的变化,从密集网络到稀疏网络的转变。我们在数值上描述了这种交叉,揭示了簇大小分布中的幂律衰减,并通过连接渗透理论进行了分析。与数据的比较表明,种群通过保持在交点附近向稀疏阶段进化。这些发现提供了对驱动细胞群体适应动态的集体组织的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


