Ndc80 complex, a conserved coupler for kinetochore-microtubule motility, is a sliding molecular clutch
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Chromosome motion at spindle microtubule plus ends relies on dynamic molecular bonds between kinetochores and proximal microtubule walls. Under opposing forces, kinetochores move bidirectionally along these walls while remaining near the ends, yet how continuous wall sliding occurs without end detachment remains unclear. Using ultrafast force-clamp spectroscopy, we show that single Ndc80 complexes, the primary microtubule-binding kinetochore component, exhibit processive, bidirectional sliding. Plus end–directed forces induce a mobile catch bond in Ndc80, increasing frictional resistance and restricting sliding toward the tip. Conversely, forces pulling Ndc80 away from the plus end trigger mobile slip-bond behavior, facilitating sliding. This dual behavior arises from force-dependent modulation of the Nuf2 calponin-homology domain’s microtubule binding, identifying this subunit as a friction regulator. We propose that Ndc80’s ability to modulate sliding friction provides the mechanistic basis for the kinetochore’s end coupling, enabling its slip-clutch behavior.
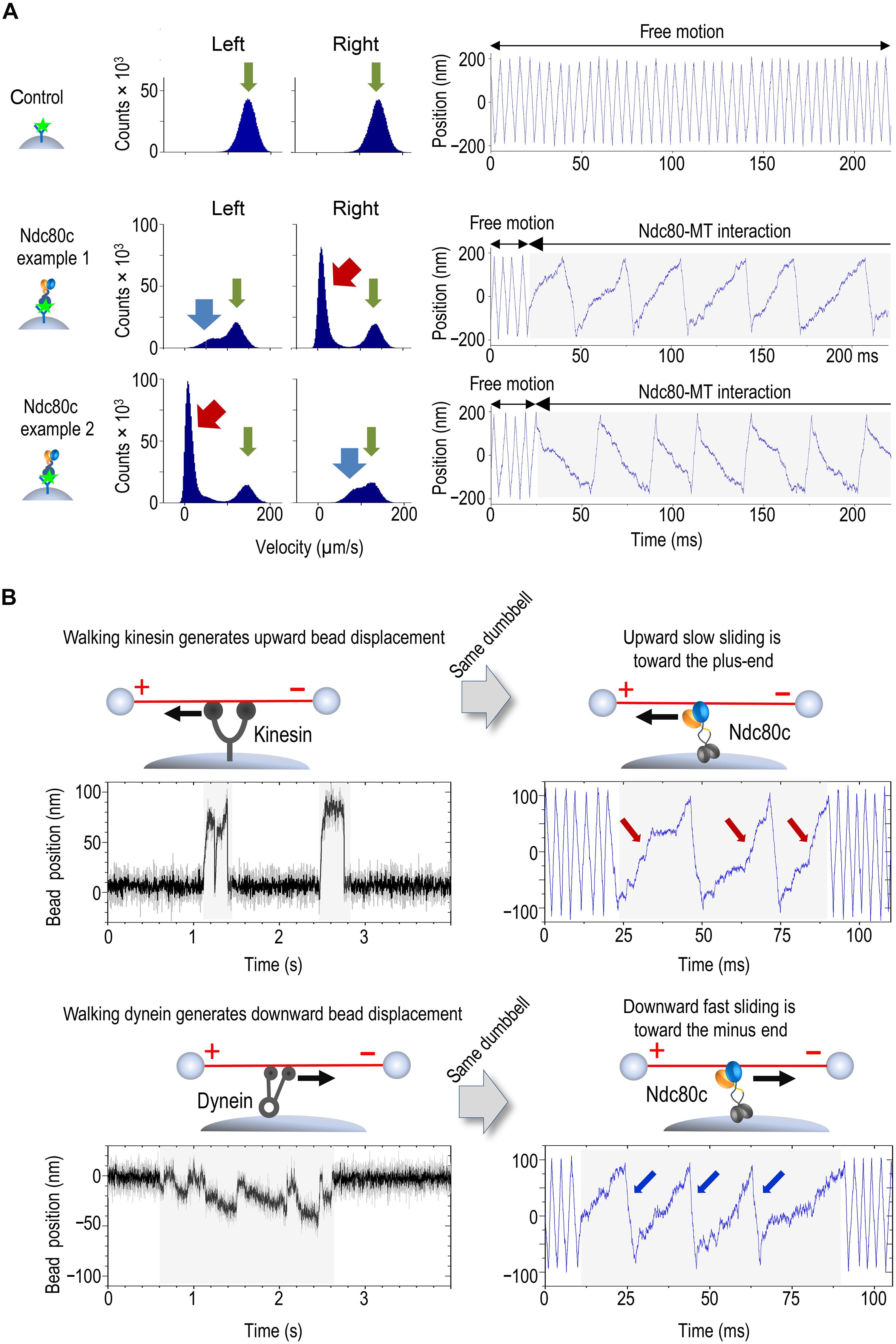
Ndc80复合物是一种滑动的分子离合器,是着丝细胞-微管运动的保守耦合器
纺锤体微管正端的染色体运动依赖于着丝点和近端微管壁之间的动态分子键。在相反的作用力下,着丝点沿着这些壁双向移动,同时保持在末端附近,但如何在没有末端分离的情况下发生连续的壁滑动尚不清楚。利用超快力钳光谱,我们发现单个Ndc80配合物,微管结合着丝点的主要成分,表现出渐进的双向滑动。再加上端向力在Ndc80中诱导了一个移动的捕获键,增加了摩擦阻力并限制了向尖端的滑动。相反,将Ndc80拉离正极的力会触发滑动粘合行为,从而促进滑动。这种双重行为源于Nuf2钙蛋白同源结构域的微管结合的力依赖性调节,确定该亚基为摩擦调节剂。我们认为Ndc80调节滑动摩擦的能力为着丝点的末端耦合提供了机制基础,从而实现了其滑动离合器行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


