Loss of CMTR1 leads to gastrulation failure and early embryonic lethality
IF 2.1
3区 生物学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
Abstract
Cap Methyltransferase 1 (CMTR1) facilitates the addition of a 5’ methyl cap on eukaryotic mRNA molecules. Using a knock-out (KO) allele, we demonstrate that CMTR1plays an essential role during gastrulation. In the absence of CMTR1, mutant embryos undergo early lethality, arresting prior to organogenesis with severe developmental delay apparent at E7.5. Multiple molecular approaches indicate significant disruptions in the ability of the CMTR1-KO embryo to form the three primary germ layers – likely driving the observed gastrulation failure. Our analysis of CMTR1 has revealed an unexpected sexually dimorphic phenotype. Female CMTR1 null embryos are more severely delayed and have increased differentially expressed genes compared to male mutants; presumably causing a variety of downstream consequences and a more severe developmental phenotype. Importantly, we do not observe defects in X-inactivation, suggesting that there are unidentified sexually dimorphic mechanisms active during early embryonic stages, prior to the onset of known differences between XX and XY embryos. In sum, we illustrate the necessity of CMTR1 during embryonic development and reveal novel insights into differences in gene regulation pathways between sexes prior to organogenesis.
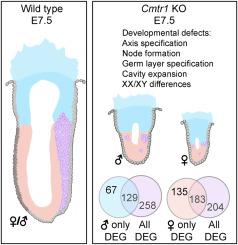
CMTR1的缺失导致原肠胚发育失败和早期胚胎死亡
帽甲基转移酶1 (CMTR1)有助于在真核mRNA分子上添加一个5 '甲基帽。使用敲除(KO)等位基因,我们证明cmtr1在原肠胚形成过程中起重要作用。在缺乏CMTR1的情况下,突变胚胎会发生早期致死,在器官发生之前发生阻滞,在E7.5时出现严重的发育迟缓。多种分子方法表明,CMTR1-KO胚胎形成三个初级胚层的能力明显中断,可能导致观察到的原肠胚形成失败。我们对CMTR1的分析揭示了一种意想不到的两性二态表型。与雄性突变体相比,雌性CMTR1缺失胚胎延迟更严重,差异表达基因增加;可能会导致各种下游后果和更严重的发育表型。重要的是,我们没有观察到x失活的缺陷,这表明在已知的XX和XY胚胎之间的差异开始之前,在胚胎早期阶段存在未识别的两性二态机制。总之,我们阐明了CMTR1在胚胎发育过程中的必要性,并揭示了器官发生前性别间基因调控途径差异的新见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental biology
生物-发育生物学
CiteScore
5.30
自引率
3.70%
发文量
182
审稿时长
1.5 months
期刊介绍:
Developmental Biology (DB) publishes original research on mechanisms of development, differentiation, and growth in animals and plants at the molecular, cellular, genetic and evolutionary levels. Areas of particular emphasis include transcriptional control mechanisms, embryonic patterning, cell-cell interactions, growth factors and signal transduction, and regulatory hierarchies in developing plants and animals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


