Persistent Chronic Thrombo-Inflammation in Anti–Glomerular Basement Membrane Disease Despite Immune Complex Removal
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
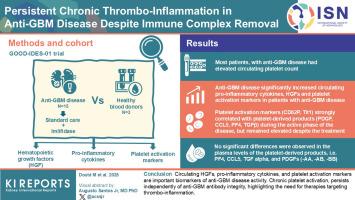
Anti–glomerular basement membrane (anti-GBM) disease is a severe autoimmune disease characterized by autoantibody-mediated glomerular damage, leading to a rapid decline in kidney function and end-stage renal disease. The stimulation of megakaryopoiesis (Mkpoiesis) and platelet production, driven by kidney-derived hematopoietic growth factors (HGFs) such as thrombopoietin (TPO), exacerbates chronic thrombosis and inflammation. Activated platelets release bioactive molecules able to promote microvascular dysfunction, cell proliferation, and excessive extracellular matrix deposition in injured glomeruli. Understanding the molecular mechanisms driving persistent thrombo-inflammation during anti-GBM disease will enhance therapeutic improvements. The hypothesis of this study was that anti-GBM disease could stimulate the production of kidney-derived HGFs and proinflammatory mediators. In addition, immobilized anti-GBM antibodies in glomeruli could directly activate FcγRIIA expressing platelets, thereby promoting chronic platelet activation during anti-GBM disease.
Methods
In the GOOD-IDES-01 trial, patients received, in addition to standard care, imlifidase (the IgG-degrading enzyme, IdeS). In plasma samples collected from patients with anti-GBM disease, before and after imlifidase treatment, and from healthy blood donors (HBDs), we analyzed plasma HGFs, proinflammatory and platelet activation markers, and platelet-derived products.
Results
Anti-GBM disease significantly elevated plasma proinflammatory and platelet activation markers, and HGFs (TPO and stem cell factor [SCF]). Plasma TPO correlated with anti-GBM titers. Standard care and imlifidase treatment only reduced TPO levels and platelet counts. Platelet activation markers (CD62P and Tlt1) strongly correlated with platelet-derived products (PDGF, CCL5, PF4, and TGFβ) during the active phase of the disease, but remained elevated despite the treatment.
Conclusion
Circulating HGFs, proinflammatory cytokines, and platelet activation markers are important biomarkers of anti-GBM disease activity. Chronic platelet activation, persists independently of anti-GBM antibody integrity, highlighting the need for therapies targeting thrombo-inflammation.
尽管免疫复合物去除,抗肾小球基底膜病仍存在持续性慢性血栓炎症
抗肾小球基底膜病(anti-GBM)是一种以自身抗体介导的肾小球损伤为特征的严重自身免疫性疾病,可导致肾功能迅速下降和终末期肾病。由肾源性造血生长因子(HGFs)如血小板生成素(TPO)驱动的巨核生成(Mkpoiesis)和血小板生成的刺激加剧了慢性血栓形成和炎症。活化的血小板释放生物活性分子,能够促进受损肾小球微血管功能障碍、细胞增殖和过度的细胞外基质沉积。了解抗gbm疾病期间驱动持续血栓炎症的分子机制将提高治疗效果。本研究的假设是抗gbm疾病可以刺激肾源性hgf和促炎介质的产生。此外,固定在肾小球内的抗gbm抗体可直接激活表达FcγRIIA的血小板,从而促进抗gbm疾病期间的慢性血小板活化。方法在GOOD-IDES-01试验中,患者除了接受标准治疗外,还接受了imlifidase (igg降解酶,IdeS)。在抗gbm患者、imlifidase治疗前后和健康献血者(HBDs)的血浆样本中,我们分析了血浆hgf、促炎和血小板活化标志物以及血小板衍生产物。结果抗gbm显著升高血浆促炎和血小板活化标志物,以及HGFs (TPO和干细胞因子[SCF])。血浆TPO与抗gbm滴度相关。标准护理和氨酰酶治疗仅能降低TPO水平和血小板计数。血小板活化标志物(CD62P和Tlt1)在疾病活动期与血小板衍生产物(PDGF、CCL5、PF4和TGFβ)密切相关,但在治疗后仍升高。结论循环hgf、促炎因子和血小板活化标志物是抗gbm疾病活性的重要生物标志物。慢性血小板活化,独立于抗gbm抗体的完整性,强调需要针对血栓炎症的治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


