Prospective Pilot Study of Plasma Cell–Targeted Therapy With Bortezomib in Refractory IgA Nephropathy
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
IgA nephropathy (IgAN) is a leading cause of kidney failure, characterized by galactose-deficient IgA1 (Gd-IgA1) deposition and immune complex formation. Aberrant trafficking of IgA+ plasma cells and autoantibody production (IgG or IgA) contribute to disease pathogenesis. Proteasome inhibitors such as bortezomib may modulate B or plasma cell activity and reduce pathogenic antibody production.
Methods
This open-label, prospective, uncontrolled trial evaluated bortezomib in adults with biopsy-confirmed IgAN, proteinuria > 1.5 g/d, and estimated glomerular filtration rate (eGFR) ≥ 30 ml/min per 1.73 m2 despite optimized care. Patients received 4 to 8 doses of i.v. bortezomib at 1.1 to 1.3 mg/m2 per dose. The primary end point was achieving 24-hour proteinuria (24 h–UP) < 300 mg/24 h at 12 months. Secondary end points included changes in eGFR and adverse event monitoring.
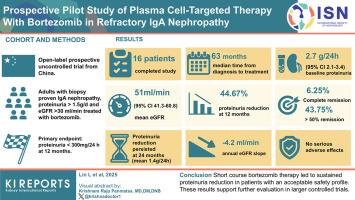
Results
Sixteen patients completed the study. Median time from diagnosis to treatment was 63 months (range: 10–192). Baseline proteinuria was 2.719 g/24 h (95% confidence interval [CI]: 2.169–3.408), and mean eGFR was 51.1 ml/min per 1.73 m2 (95% CI: 41.3–60.8). At 12 months, proteinuria decreased by 44.67%, with 6.25% achieving complete remission and 43.75% achieving ≥ 50% reduction. Proteinuria reduction persisted at 24 months (mean: 1.411 g/24 h: 48.09% reduction). The annual eGFR slope was −4.275 ml/min per 1.73 m2. No serious treatment-related adverse events were reported.
Conclusion
Short course bortezomib therapy led to sustained proteinuria reduction in patients with IgAN, with an acceptable safety profile. These results support further evaluation in larger, controlled trials.
硼替佐米治疗难治性IgA肾病的血浆细胞靶向治疗的前瞻性先导研究
iga肾病(IgAN)是肾衰竭的主要原因,其特征是半乳糖缺乏IgA1 (Gd-IgA1)沉积和免疫复合物形成。异常运输的IgA+浆细胞和自身抗体的产生(IgG或IgA)有助于疾病的发病。蛋白酶体抑制剂如硼替佐米可调节B细胞或浆细胞活性,减少致病性抗体的产生。方法:这项开放标签、前瞻性、非对照试验评估了硼替佐米对活检证实IgAN、蛋白尿≥1.5 g/d、肾小球滤过率(eGFR)≥30 ml/min / 1.73 m2的成人的疗效,尽管有优化的护理。患者接受4至8次静脉滴注硼替佐米,每次剂量1.1至1.3 mg/m2。主要终点是12个月时达到24小时蛋白尿(24 h - up) < 300 mg/24 h。次要终点包括eGFR变化和不良事件监测。结果16例患者完成了研究。从诊断到治疗的中位时间为63个月(范围:10-192个月)。基线蛋白尿为2.719 g/24 h(95%可信区间[CI]: 2.169-3.408),平均eGFR为51.1 ml/min / 1.73 m2 (95% CI: 41.3-60.8)。12个月时,蛋白尿下降44.67%,其中6.25%达到完全缓解,43.75%达到≥50%的减少。蛋白尿持续减少24个月(平均:1.411 g/24小时:48.09%减少)。年eGFR斜率为- 4.275 ml/min / 1.73 m2。没有严重的治疗相关不良事件的报道。结论:短期硼替佐米治疗可使IgAN患者持续减少蛋白尿,并具有可接受的安全性。这些结果支持在更大规模的对照试验中进一步评价。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


